Results for “reciprocity” 39 found
UK to Adopt Pharmaceutical Reciprocity!
More than twenty years ago I wrote:
If the United States and, say, Great Britain had drug-approval reciprocity, then drugs approved in Britain would gain immediate approval in the United States, and drugs approved in the United States would gain immediate approval in Great Britain. Some countries such as Australia and New Zealand already take into account U.S. approvals when making their own approval decisions. The U.S. government should establish reciprocity with countries that have a proven record of approving safe drugs—including most west European countries, Canada, Japan, and Australia. Such an arrangement would reduce delay and eliminate duplication and wasted resources. By relieving itself of having to review drugs already approved in partner countries, the FDA could review and investigate NDAs more quickly and thoroughly.
Well, it’s happening! After Brexit, there were concerns that drugs would take longer to get approved in the UK because the EU was a much larger market. To address this, the UK introduced the “reliance procedure” which recognized the EU as a stringent regulator and guaranteed approval in the UK within 67 days for any drug approved in the EU. The Reliance Procedure essentially kept the UK in the pre-Brexit situation, and was supposed to be temporary. However, recognizing the logic of recognizing the EU, the UK is now saying that it will recognize other countries.
Our aim is to extend the countries whose assessments we will take account of, increasing routes to market in the UK. We will communicate who these additional regulators are and publish detailed guidance about this new framework in due course, including any transition arrangements for applications received under existing frameworks.
The UK is already participating in a mutual recognition agreement with the FDA over some cancer drugs. Therefore, it seems likely that the FDA will be among the regulatory authorities that the UK recognizes. If the UK does recognize the FDA, then we only need the FDA to recognize the UK for my scenario from more than 20 years ago to be fulfilled.
It’s thus time to revisit the Lee-Cruz bill of 2015, which proposed the Result Act (I was an influence).
Reciprocity Ensures Streamlined Use of Lifesaving Treatments Act (S. 2388), or the RESULT Act,” which would amend the Food, Drug and Cosmetic Act to allow for reciprocal approval of drugs.
Addendum: Many previous posts on FDA reciprocity.
Do you have reciprocity anxiety?
Namely the fear of owing other people, or institutions, a favor, or maybe just the possible perception of such?:
The researchers believe reciprocity anxiety is likely to be greater the bigger a favour and the more public its receipt. They think it’s a trait that companies should take an interest in – while loyalty schemes, vouchers and other freebies have obvious appeal to many customers, results from two initial studies suggested that these marketing strategies are actually likely to deter others…
In a follow-up study, volunteers imagined a shop attendant offering them a free drink and plate full of snacks. Afterwards, high scorers in reciprocity anxiety scored lower for customer satisfaction and they said they would be less willing to visit the store again and less willing to spread a good word about the shop.
“Reciprocity works to establish a psychological bond” between customer and firm, the researchers said, but the discomfort it causes can backfire among those high in reciprocity anxiety, especially if they feel the benefits reflect badly on them or that they will struggle to reciprocate (around 18 per cent of people tested in these new studies scored highly in the trait; age and gender were unrelated).
Here is the full article, and the pointer is from Michelle Dawson. Finally:
…I wonder how it might impact the ways that people manage their friendships and other relationships – perhaps high scorers in reciprocity anxiety are inclined to turn down invitations, seek help or receive other friendly favours, putting them at risk of loneliness and isolation.
Pharmaceutical Reciprocity for Canada
If pharmaceutical reciprocity is a good idea for the United States, it’s a great idea for smaller countries. Indeed, this is mostly what happens in practice, even if not by law, since smaller countries can’t afford or justify the expensive US process. Fred Roeder of the Montreal Economic Institute makes the case for reciprocity in Canada:
…reciprocal recognition of drug approval authorities on both sides of the Atlantic would incentivize Canadian, European and American authorities to spend less time and money conducting parallel reviews. If the HPFB, the FDA or the EMA approved a drug, patients in Canada, Europe and America would have immediate access to it — increasing consumers’ choice as new drugs are offered to patients faster and more affordably, with less red tape driving up costs.
A reduction in approval time can be a win-win for patients and firms because a decrease in approval time is an increase in effective patent length without an actual increase in patent length. The numbers below are optimistic, but the idea that streamlining approval can increase profits and stimulate investment is correct:
These market-oriented reforms would not benefit not only consumers, but the pharmaceutical companies as well, expanding the timespan of the patents. On average, new drugs have a mere 10 to 14 years of patent protection remaining by the time they are sold to consumers after they have successfully jumped over all the government hurdles. Streamlining the drug approval process would increase the timespan of patented drugs on the market by 50 to 70 per cent.
Roeder also mentions Bart Madden’s important book Free to Choose Medicine. (For those who don’t know, I am proud to be the Bartley J. Madden chair in economics at Mercatus at GMU.)
Reciprocity and Muscular Dystrophy
For years, muscular dystrophy patients in the United States have been purchasing the drug deflazacort — used to stabilize muscle strength and keep patients mobile for a period of time — from companies in the United Kingdom at a manageable price of $1,600 a year.
But because an American company just got approval from the Food and Drug Administration to sell the drug in the United States, the price of the drug will soar to a staggering $89,000 annually, the Wall Street Journal reported last week.
Because the FDA restricts the importing of drugs from overseas if a version is available domestically, patients are stuck with the new, expensive version. This makes deflazacort the perfect case for advocates of international drug reciprocity — a reform that would make it easier for consumers to buy drugs that have been approved in other developed countries.
That is the introduction to an interview with yours truly in the Washington Post. I discuss thalidomide and the race to the bottom argument. Here is one other bit:
IT: Do you have any thoughts about the potential for FDA reform under this new administration and Congress?
AT: Peter Thiel’s speech at the Republican National Convention reminded us that we used to take big, bold risks — like going to the moon. Today, to say a project is a “moon shot” is almost a put-down, as if going to the moon never happened. We have become risk-averse and complacent, to borrow a term from my colleague Tyler Cowen. The result of the incessant focus on safety is playgrounds without teeter totters, armed guards at our schools and national monuments, infrastructure projects that no longer get built, and pharmaceutical breakthroughs that never happen.
The new administration is unpredictable, but when it comes to the FDA, unpredictable is better than business as usual.
The administration has yet to appoint a great FDA commissioner. Early names floated included Balaji Srinivasan, Jim O’Neill, Joseph Gulfo, and Scott Gottlieb but Srinivasan seems to have removed himself from the running. O’Neill would be great but I don’t think the US is ready, so that leaves Gulfo and Gottlieb. My suspicion is that Trump will like Gulfo because of Gulfo’s entrepreneurial experience but, as I said, the new administration is unpredictable.
Drug Reciprocity with Europe Gains Support
As loyal readers know, I’ve long been in favor of a system where a drug approved in another major, developed country is also approved here. For a long time it seemed as if I was shouting in the wilderness but in the last few years support for the idea has grown, as the Cruz-Lee Reciprocity bill indicates. In A Cure for Swelling Drug Prices: Competition, Greg Ip at the WSJ notes another new development:
Mr. Tabarrok says the FDA should also offer reciprocal approval of drugs that regulators in other advanced countries have already cleared. Imports of generics from countries with government-negotiated prices ought not to be as controversial as patent-protected drugs because they involve far less expensive and risky research. Indeed, the Generic Pharmaceutical Association and its European equivalent, Medicines for Europe, have proposed a “single development pathway” under which approval in one jurisdiction would automatically confer approval in the other.
The proposed plan is for generics only where the issues are simpler but Greg is right to conclude more generally:
The FDA has long insisted, for safety reasons, that it approve all drugs regardless of whether they have been approved overseas. But if the FDA was once a better regulator than its overseas peers, it isn’t now. Ken Kaitin, a professor of medicine at Tufts University who has studied drug regulation around the world, says there is “absolutely no evidence” the U.S. drug supply is safer than in Britain, Canada or Europe.
Thus, the FDA wouldn’t be compromising safety by harmonizing its approvals with foreign regulators. Indeed, by making more drugs available at lower cost, it could ultimately make Americans healthier.
Economists on FDA Reciprocity
Daniel Klein & William Davis surveyed economists about whether it would be an improvement to reform the FDA so that “as soon as a new drug is approved by any one of five [FDA approved international] agencies, that drug automatically gains approval in the United States.” They report:
Of the 467 economists who answered the question and did not mark “Have no opinion,” 53 percent agreed that the reform would be an improvement, while 29 percent disagreed. (The remainder said they were “neutral.”) Moreover, those favoring the reform were more likely to say they held their belief “strongly.” Hence, the balance of economist judgment certainly leaned in favor of the liberalization.
Economists are not the only ones in favor of reciprocity. Others are also coming around, at least partially. In Generic Drug Regulation and Pharmaceutical Price-Jacking I argued in response to the massive increases in the price of Daraprim (generic name Pyrimethamine) that we ought to allow importation:
Pyrimethamine is also widely available in Europe. I’ve long argued for reciprocity, if a drug is approved in Europe it ought to be approved here. In this case, the logic is absurdly strong. The drug is already approved here! All that we would be doing is allowing import of any generic approved as such in Europe to be sold in the United States.
In a paper in JAMA discussing the same case, Drs Jeremy Greene, Gerard Anderson, and Joshua M. Sharfstein agree, writing:
A second option is to temporarily permit the importation of drug products reviewed by competent regulatory authorities and approved for sale outside the United States. For example, Glaxo, the original manufacturer of pyrimethamine, sells a version of the drug approved for use in the United Kingdom at less than $1 per tablet.
Dr Sharfstein by the way was Principal Deputy Commissioner of the US Food and Drug Administration from March 2009 to January 2011.
Addendum: I will be discussing/debating pharmaceutical policy with Dr. Sharfstein at on event sponsored by the Council on Foreign Relations in Washington, DC the morning of Monday January 25. Invitation only but email me if you want an invite.
Senators Cruz and Lee Introduce Reciprocity Bill
Senators Ted Cruz (R-Texas) and Mike Lee (R-Utah) have just introduced a bill that would implement an idea that I have long championed, making drugs, devices and biologics that are approved in other developed countries also approved for sale in the United States. Highlights of the “Reciprocity Ensures Streamlined Use of Lifesaving Treatments Act (S. 2388), or the RESULT Act,” include:
- Amending the Food, Drug and Cosmetic Act to allow for reciprocal approval of drugs, devices and biologics from foreign sponsors in certain trusted, developed countries including EU member countries, Israel, Australia, Canada and Japan.
- Encouraging the FDA to expeditiously review life-saving drug and device applications, this legislation would provide the FDA with a 30-day window to approve or deny a sponsor’s application….
- The HHS Secretary is instructed to approve a drug, device or biologic if the FDA confirms the product is:
- Lawfully approved for sale in one of the listed countries;
- Not a banned device by current FDA standards;
- There is a public health or unmet medical need for the product.
- If a promising application for a life-saving drug is declined Congress is granted the authority to disapprove of a denied application and override an FDA decision with a majority vote via a joint resolution.
In explaining why he introduced the bill Senator Cruz argued:
We continue to lose far too many of our loved ones to the “invisible graveyard,” as economist Alex Tabarrok has described: lives that could have been saved but for a bureaucratic barrier that rejects medical cures and innovation…The bill I am introducing takes the first step to reverse this trend. It provides for reciprocal drug approval, so that cures and medical devices that are already approved in other countries can more expeditiously come to the U.S.
The FDA and International Reciprocity
Bacterial meningitis causes swelling of the membranes covering the brain and spinal cord. In the United States the disease kills approximately 500 people a year, often within days of infection. Survivors can have permanent disabilities including paralysis and mental disabilities. Since March seven cases of the type B strain have been diagnosed at Princeton University, with one case just last week. A vaccine exists and is available in Europe and Australia but the FDA has not permitted the type B vaccine for use in the United States.
The Centers for Disease Control and Prevention, however, has lobbied the FDA and they have now received special and unusual permission to import the type B vaccine. Following the CDCs recommendation, Princeton University has agreed to administer and pay for the vaccine for any student that wants it.
It’s good that the FDA has lifted the ban on the type B vaccine but why should Americans have to wait for the FDA? Americans living in Europe or Australia can be prescribed the vaccine so why not here? I believe that Americans should have the right to be prescribed any drug that has been approved in Europe, Australia, Canada, Japan or other developed nation.
Indeed, as Dan Klein and I wrote at FDAReview.org, international reciprocity of drug approvals is simple common sense:
If the United States and, say, Great Britain had drug-approval reciprocity, then drugs approved in Britain would gain immediate approval in the United States, and drugs approved in the United States would gain immediate approval in Great Britain. Some countries such as Australia and New Zealand already take into account U.S. approvals when making their own approval decisions. The U.S. government should establish reciprocity with countries that have a proven record of approving safe drugs—including most west European countries, Canada, Japan, and Australia. Such an arrangement would reduce delay and eliminate duplication and wasted resources. By relieving itself of having to review drugs already approved in partner countries, the FDA could review and investigate NDAs more quickly and thoroughly.
As has now become clear, international reciprocity is not just about choice it can also save lives.
In Conversation with Próspera CEO Erick Brimen & Vitalia Co-Founder Niklas Anzinger
During my visit to Prospera, one of Honduras’ private governments under the ZEDE law, I interviewed Prospera CEO Erick Brimen and Vitalia co-founder Niklas Anzinger. I learned a lot in the interview including the real history of the ZEDE movement (e.g. it didn’t begin with Paul Romer). I also had not fully appreciated the power of reciprocity stacking.
Companies in Prospera have the unique option to select their regulatory framework from any OECD country, among others. Erick Brimen elaborated in the podcast how this enables companies to do normal, OECD approved, things in Prospera which literally could not be done legally anywhere else in the world.
…so in the medical world for instance you have drugs that are approved in some countries but not others and you have medical practitioners that are licensed in some countries but not the others and you have medical devices approved in some countries but not others and there’s like a mismatch of things that are approved in OECD countries but there’s no one location where you can say hey if they’re approved in any country they’re approved here. That is what Prosper is….Our hypothesis is that just by doing that we can leapfrog to a certain extent and it’s got nothing to do with the wild west or doing weird things.
…so here so you can have a drug approved in the UK but not in the US with a doctor licensed in the US but not in the UK with a medical device created in Israel but not yet approved by the FDA following a procedure that has been say innovated in Canada, all of that coming together here in Prospera.
Speeding Up Pharmaceutical Approvals by Recognizing Other Stringent Regulators
New Zealand’s ACT party has proposed that New Zealand speed up pharmaceutical approvals by recognizing the decisions of other stringent regulators, an idea I have long promoted .
The average time for Medsafe to consent an application for a high risk medicine is 630 days. For intermediate risk, it is 661 days and for lower risk it is 830 days8. The average time taken just for processing some lower risk categories is 176-210 days. This is an unacceptable length of time, given there other regulatory bodies replicating that exact same work overseas.
ACT says if a drug or medical device has been approved by any two reputable foreign regulatory bodies (such as Australia, United States, United Kingdom), it should be automatically approved in NZ as well within one week unless Medsafe can show extraordinary reason why it shouldn’t be.
This simple change would significantly improve access to medicines that have already been subject to rigorous testing and analysis through other regulatory regimes.
The ACT party is small but it has some seats and surprisingly the much larger National party is proposing a similar rule:
New Zealand’s slow approval process for medicines means Kiwis wait much longer than people in other countries to access potentially life-saving treatments. While it is essential that medicines and other treatments are subject to stringent scrutiny to ensure they are safe, there is no reason why New Zealanders should have to wait for our domestic medicines regulatory body, Medsafe, to conduct its own cumbersome process from scratch, when countries with health systems we trust have already gone through this exercise.
National will:…• Require Medsafe to implement even faster approvals processes for any medicines for use in New Zealand that have already been approved by at least two regulatory bodies that we currently recognise, including Australia, the EU, Singapore, the UK, Switzerland and the US.
New Zealand, by the way, already has a reciprocity agreement with the United States for food and it’s mutual–the FDA also recognizes New Zealand as a stringent food regulator–so the idea is not unprecedented.
Moreover, all of this comes on the tail of the UK actually adopting the idea via the “reliance procedure” which recognizes the EU as a stringent regulator and guarantees approval in the UK within 67 days for ay drug approved in the EU.
In the United States, even AOC has flirted with the idea, at least for sunscreens!
Thus, the reciprocity or recognition idea is starting to be adopted.
Hat tip: Eric Crampton who has some further comments.
AOC Gets on the Anti-FDA Bandwagon
At least when it comes to suncreen. As long-time readers will know, I have been complaining about FDA over-regulation of sunscreen for a decade! Maybe now that AOC is on the case things will change.
AOC’s sunscreen video is pretty good. One point she doesn’t stress is that requiring Americans to use more oily, less natural-feeling sunscreen can cause less use and thus more skin cancer. Even more important is the general issue of reciprocity or polycentric authority:
My rule is very simple. I don’t think the FDA is better than the EMA so if any drug or device is approved in Europe it ought to be available for purchase in the United States with a label saying “Approved by the EMA. Not approved by the FDA.” (By the way, we do have reciprocity type agreements with Canada and New Zealand for food so this would not be unprecedented.)
US sunscreens are far behind the rest of the world and our regulations aren’t necessarily making our sunscreens better or safer — but it doesn’t have to be this way! pic.twitter.com/vaZXpZ2a7S
— Rep. Alexandria Ocasio-Cortez (@RepAOC) August 11, 2023
A Pox on the FDA
Monkeypox isn’t in the same category of risk that COVID was before vaccines but it’s a significant risk, especially in some populations, and it’s a test of how much we have learned. The answer is not bloody much. Here’s James Walsh in NYMag:
As monkeypox cases have ticked up nationwide, the White House and federal agencies have repeatedly assured the public that millions of vaccine doses will be distributed to at-risk populations before the end of the year. Yet since the World Health Organization announced the global monkeypox outbreak in May, only tens of thousands of shots have been administered in the U.S. The slow start is due, at least in part, to the fact that 1.1 million doses have been stored in a Denmark pharmaceutical facility while the Food and Drug Administration has taken almost two months to approve their release here, according to people familiar with the situation. FDA officials only began to inspect the facility last week. The lag time, public-health experts say, is indicative of the federal government’s lackadaisical approach to a growing public-health emergency.
…It’s unclear why the FDA took so long to send inspectors to Denmark. The agency regularly conducted virtual inspections of drug facilities early in the COVID-19 pandemic, according to the agency’s guidance, and public-health activists are demanding answers. “Members of at risk communities are being turned away from monkeypox vaccination because these vaccines are not available in sufficient quantity in the U.S., but instead sitting in freezers in Denmark,” members of the advocacy group PrEP4All and Partners in Health wrote in a letter to federal officials overseeing the outbreak response last week.
Compounding their frustrations was the FDA’s refusal to accept an inspection done last year by its counterpart, the European Medicines Agency, which deemed the company’s facility in compliance with the FDA’s own standards.
“The FDA does not grant reciprocity for EMA authorization of any vaccines, for monkeypox or other diseases,” a spokesperson for the FDA said in a statement.
Is there anyone in the United States who is saying, “I am at risk of Monkeypox and I want the vaccine but I don’t trust the European Medicines Agency to run the inspection. I’d rather wait for the FDA!” I don’t think so. James Krellenstein, an activist on this issue, asks:
“Why were the Europeans able to inspect this plant a year ago, ensuring these doses can be used in Europe and the Biden Administration didn’t do the same,” he added. “The FDA is making a judgment that they’d rather let gay people remain unvaccinated for weeks and weeks and weeks than trust the European certification process.”
Many people want to be vaccinated:
New York City has received just 7,000 doses from the federal government amid the national vaccine shortage. Meanwhile, the city Department of Health and Mental Hygiene’s appointment booking system has failed to keep up with the high demand for the shots — most recently on Wednesday.
…The mounting frustrations left health officials and Mayor Eric Adams on the defensive, pushing back against comparisons to New York’s struggles during the early days of the coronavirus vaccine, which was beset by computer glitches and supply shortages.
This is a classic case for reciprocity. Any drug, vaccine, test or sunscreen (!) approved by a stringent regulatory authority ought to be conditionally approved in the United States.
Addendum: If you are not furious already–and you should be–remember that during COVID the FDA suspended factory inspections around the world creating shortages of life-saving cancer drugs and other pharmaceuticals. As I wrote then “Grocery store workers are working, meat packers are working, hell, bars and restaurants are open in many parts of the country but FDA inspectors aren’t inspecting. It boggles the mind.”
Hat tip: Josh Barro.
Photo Credit: Nigeria Centre for Disease Control.
The FDA is Increasing Skin Cancer
Americans who travel to the beaches in France, Spain, or Italy routinely do something that is illegal in the United States–they buy and use European sunscreens to protect themselves from sunburn and skin cancer. Suncreens in Europe and Asia are better than in the United States because more ingredients are allowed and these create more effective and more pleasing suncreens. I’ve been writing about this since 2013! My view hasn’t changed:
My rule is very simple. I don’t think the FDA is better than the EMA so if any drug or device is approved in Europe it ought to be available for purchase in the United States with a label saying “Approved by the EMA. Not approved by the FDA.” (By the way, we do have reciprocity type agreements with Canada and New Zealand for food so this would not be unprecedented.)
Here’s the latest from Amanda Mull writing in the Atlantic:
Newer, better UV-blocking agents have been in use in other countries for years. Why can’t we have them here?
…In formal statements and position papers, doctors and cancer-prevention advocates express considerable interest in bringing new sunscreen ingredients to the American market, but not a lot of optimism that any will be available soon.
…In 2014, Congress passed a law attempting to speed access to sunscreen ingredients that have been in wide use in other countries for years, but it hasn’t really worked. “The FDA was supposed to be fast-tracking these ingredients for approval, because we have the safety data and safe history of usage from the European Union,” Dobos said. “But it seems to continually be stalled.” According to Courtney Rhodes, a spokesperson for the FDA, manufacturers have submitted eight new active ingredients for consideration. The agency has asked them to provide additional data in support of those applications, but none of them has yet satisfied the agency’s requirements.
“In the medical community, there is a significant frustration about the lack of availability of some of the sunscreen active ingredients,” Henry Lim, a dermatologist at Henry Ford Health, in Michigan, told me. The more filters are available to formulators, the more they can be mixed and matched in new ways, which stands to improve not just the efficacy of the final product, but how it feels and looks on your skin, and how easy it is to apply. On a very real level, making sunscreen less onerous to use can make it more effective. “The best sunscreen is going to be the one you’re going to use often and according to the directions,” Dobos said. Skin cancer is the most common type of cancer in the United States, and by one estimate, one in five Americans will develop it in their lifetime.
Hat tip: Joe.
Infant Formula, Price Controls, and the Misallocation of Resources
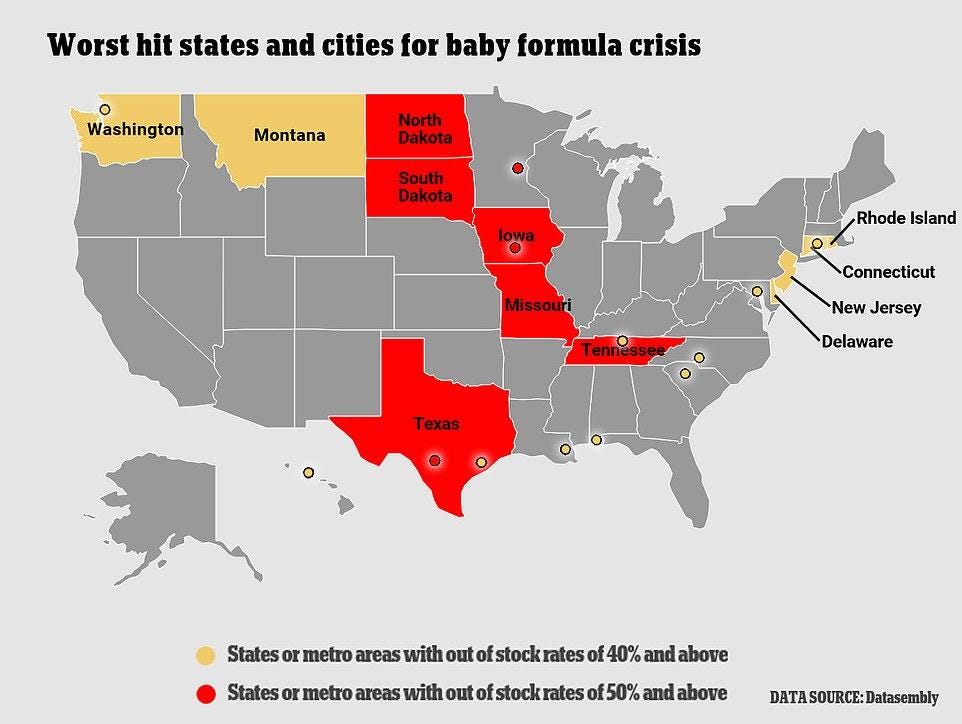 I’ve been reluctant to write about the shortage of infant formula simply because it’s so tiring to say the same thing over and over again. Obviously, this is a classic case where the FDA should allow imports of any food or baby formula approved by a stringent authority. (Here’s the US Customs and Border Patrol bragging about how they nabbed 588 cases of infant formula from Germany and the Netherlands as if it were cocaine.) Scott Lincicome has an excellent run down which covers not just the FDA but the problems caused by trade regulation and the WIC program as well.
I’ve been reluctant to write about the shortage of infant formula simply because it’s so tiring to say the same thing over and over again. Obviously, this is a classic case where the FDA should allow imports of any food or baby formula approved by a stringent authority. (Here’s the US Customs and Border Patrol bragging about how they nabbed 588 cases of infant formula from Germany and the Netherlands as if it were cocaine.) Scott Lincicome has an excellent run down which covers not just the FDA but the problems caused by trade regulation and the WIC program as well.
What I want to do is focus on something less discussed: Why does the shortage vary across the country and even city by city?
I believe one reason is implicit price controls, either due to fear of regulatory backlash, regulatory constraints through other programs, or a misplaced desire not to upset consumers.
Price controls create shortages–that much is well known–but they also create a misallocation of goods. No doubt you have seen pictures from the 1970s of long lines of cars waiting to get gasoline. But there weren’t lineups everywhere at all times–rather we had the strange situation where there were shortage of gasoline in some places while, just a hundred miles away, there was plenty. Or shortages one day and surpluses the next.
Prices rationally allocate goods across space and time in response to shifts in demand and supply. If demand increases in one place, for example, prices rise, creating an incentive to bring in supplies from elsewhere. A rising price signals where supplies are needed and creates an incentive to deliver. Or, as Tyler and I put it, A price is a signal wrapped up in an incentive. A price controlled below the market price creates a shortage and it also kills the signaling and incentive function of prices. The result is allocational chaos: Shortages in some places and times and excess supply in other places and times.
In fact, price controls in a capitalist economy give you a window onto a planned economy. If you think of communism as a system of universal price controls this allocation chaos is the essence of why a communist state cannot rationally allocate resources.
Tyler and I discuss allocational chaos in our chapter on price controls in Modern Principles of Economics. See also this excellent video.
The prisoner’s dilemma for prisoners and Mafia men
We develop experimental evidence on cooperation and response to sanctions by running prisoner’s dilemma and third party punishment games on three different pools of subjects; students, ordinary criminals and Camorristi (Neapolitan ‘Mafiosi’). The latter two groups were recruited from within prisons. Camorra prisoners show a high degree of cooperativeness and a strong tendency to punish defectors, as well as a clear rejection of the imposition of external rules even at significant cost to themselves. The subsequent econometric analysis further enriches our understanding demonstrating inter alia that individuals’ locus of control and reciprocity are associated with quite different and opposing behaviours amongst different participant types; a strong sense of self-determination and reciprocity both imply a higher propensity to punish for Camorra inmates, but quite the opposite for ordinary criminals, further reinforcing the contrast between the behaviour of ordinary criminals and the strong internal mores of Camorra clans.
Here is the paper by Annamarie Nese, et.al., via Ethan Mollick and Ilya Novak.