Results for “first doses” 105 found
First Doses First? — show your work!
Alex has been arguing for a “First Doses First” policy, and I find his views persuasive (while agreeing that “halfsies” may be better yet, more on that soon). There are a number of numerical attempts to show the superiority of First Doses First, here is one example of a sketched-out argument, I have linked to a few others in recent days, or see this recent model, or here, here is an NYT survey of the broader debate. The simplest numerical case for the policy is that 2 x 0.8 > 0.95, noting that if you think complications overturn that comparison please show us how. (Addendum: here is now one effort by Joshua Gans).
On Twitter I have been asking people to provide comparable back-of-the-envelope calculations against First Doses First. What is remarkable is that I cannot find a single example of a person who has done so. Not one expert, and at this point I feel that if it happens it will come from an intelligent layperson. Nor does the new FDA statement add anything. As a rational Bayesian, I am (so far) inferring that the numerical, expected value case against First Doses First just isn’t that strong.
Show your work people!
One counter argument is that letting “half-vaccinated” people walk around will induce additional virus mutations. Florian Kramer raises this issue, as do a number of others.
Maybe, but again I wish to see your expected value calculations. And in doing these calculations, keep the following points in mind:
a. It is hard to find vaccines where there is a recommendation of “must give the second dose within 21 days” — are there any?
b. The 21-day (or 28-day) interval between doses was chosen to accelerate the completion of the trial, not because it has magical medical properties.
c. Way back when people were thrilled at the idea of Covid vaccines with possible 60% efficacy, few if any painted that scenario as a nightmare of mutations and otherwise giant monster swarms.
d. You get feedback along the way, including from the UK: “If it turns out that immunity wanes quickly with 1 dose, switch policies!” It is easy enough to apply serological testing to a control group to learn along the way. Yes I know this means egg on the face for public health types and the regulators.
e. Under the status quo, with basically p = 1 we have seen two mutations — the English and the South African — from currently unvaccinated populations. Those mutations are here, and they are likely to overwhelm U.S. health care systems within two months. That not only increases the need for a speedy response, it also indicates the chance of regular mutations from the currently “totally unvaccinated” population is really quite high and the results are really quite dire! If you are so worried about hypothetical mutations from the “half vaccinated” we do need a numerical, expected value calculation comparing it to something we already know has happened and may happen yet again. When doing your comparison, the hurdle you will have to clear here is very high.
When you offer your expected value calculation, or when you refuse to, here are a bunch of things you please should not tell me:
f. “There just isn’t any data!” Do read that excellent thread from Robert Wiblin. Similar points hold for “you just can’t calculate this.” A decision to stick with the status quo represents an implicit, non-transparent calculation of sorts, whether you admit it or not.
g. “This would risk public confidence in the vaccine process.” Question-begging, but even if true tell us how many expected lives you are sacrificing to satisfy that end of maintaining public confidence. This same point applies to many other rejoinders. It is fine to cite additional moral values, but then tell us the trade-offs with respect to lives. Note that egalitarianism also favors First Doses First.
h. “We shouldn’t be arguing about this, we should be getting more vaccines out the door!” Yes we should be getting more vaccines out the door, but the more we succeed at that, as likely we will, the more important this dosing issue will become. Please do not try to distract our attention, this one would fail in an undergraduate class in Philosophical Logic.
i. Other fallacies, including “the insiders at the FDA don’t feel comfortable about this.” Maybe so, but then it ought to be easy enough to sketch for us in numerical terms why their reasons are good ones.
j. All other fallacies and moral failings. The most evasive of those might be: “This is all the more reason why we need to protect everyone now.” Well, yes, but still show your work and base your calculations on the level of protection you can plausibly expect, not on the level of protection you are wishing for.
At the risk of venturing into psychoanalysis, it is hard for me to avoid the feeling that a lot of public health experts are very risk-averse and they are used to hiding behind RCT results to minimize the chance of blame. They fear committing sins of commission more than committing sins of omission because of their training, they are fairly conformist, they are used to holding entrenched positions of authority, and subconsciously they identify their status and protected positions with good public health outcomes (a correlation usually but not always true), and so they have self-deceived into pursuing their status and security rather than the actual outcomes. Doing a back of the envelope calculation to support their recommendation against First Doses First would expose that cognitive dissonance and thus it is an uncomfortable activity they shy away from. Instead, they prefer to dip their toes into the water by citing “a single argument” and running away from a full comparison.
It is downright bizarre to me — and yes scandalous — that a significant percentage of public health experts are not working day and night to produce and circulate such numerical expected value estimates, no matter which side of the debate they may be on.
How many times have I read Twitter threads where public health experts, at around tweet #11, make the cliched call for transparency in decision-making? If you wish to argue against First Doses First, now it is time to actually provide such transparency. Show your work people, we will gladly listen and change our minds if your arguments are good ones.
The problem with fitting third doses into a regulatory structure
That is a key theme of my latest Bloomberg column, here is one excerpt:
In the U.S., President Joe Biden’s administration is now pushing third booster shots for people who already have been vaccinated. That might be a good idea, but it too creates additional uncertainty for travel and migration — and for social interaction more broadly. If three doses are so important, should people be allowed to travel (or for that matter interact indoors) with only two doses? The bar is raised yet again.
Of course the issues do not end with the third dose. If the efficacy of the second dose declines significantly in less than a year, might the same happen with the third dose? How long before four doses are necessary, or maybe five? Or what if yet another significant Covid variant comes along, and only some people have a booster dose against that strain? What then counts as being “sufficiently vaccinated”?
Many Americans seem to be keen to get their third dose, but by the nature of counting that number is fewer than the number willing to get two doses. Furthermore, many people might just tire of the stress of dealing with an ongoing stream of obligatory booster shots and stop at one or two.
The sad reality is that the “two-dose standard” may not last very long, whether abroad or domestically (the same is true of the even weaker one-dose standard with Johnson & Johnson and AstraZeneca). Vaccine mandates will become harder to define and enforce, will be less transparent, and will probably be less popular.
If you tell people that three doses are needed for safety, but two doses are enough to get you into a concert or government building, how are they supposed to sort out the mixed messages? It is not obvious that enough people will get the third dose in a timely manner to make that a workable standard for vaccine passports.
Add to that the problems with the Johnson & Johnson vaccine, which originally the government urged people to get. Now those people are not being given comparable chances to obtain boosters — in fact, they are not yet being given specific guidance at all. Are they orphaned out of any new vaccine passport system, or will (supposedly dangerous?) exceptions be made for them? Or do they just have to start all over?
The big international winner from all this is likely to be Mexico, which has remained an open country and is not relying on vaccine passports. In general I do not admire Mexico’s lackadaisical Covid response, but the country may end up in a relatively favorable position, most of all when it comes to tourism and international business meetings.
As for the U.S. and Europe, the temptation to escalate required safety measures is understandable. But the previous vaccine standards were largely workable ones. If they are made tougher, they might break down altogether.
Recommended.
Second Doses Are Better at 8 Weeks or Longer
In Britain people are now being warned *not* to get their second dose at 3 or 4 weeks because this offers less protection than waiting 8 weeks or longer.
Warnings over the lack of long-term protection offered by jab intervals shorter than eight weeks come as scores of under 40s continue to receive second doses early at walk-in clinics, contrary to Government guidance.
…“There is very good immunological and vaccine effectiveness evidence that the longer you leave that second dose the better for Pfizer and eight weeks seems to be a reasonable compromise.”
Professor Harnden emphasised that “you’re definitely less protected against asymptomatic disease if you have a shorter dose interval”.
I’m so old I can remember when first doses first wasn’t “following the science.”
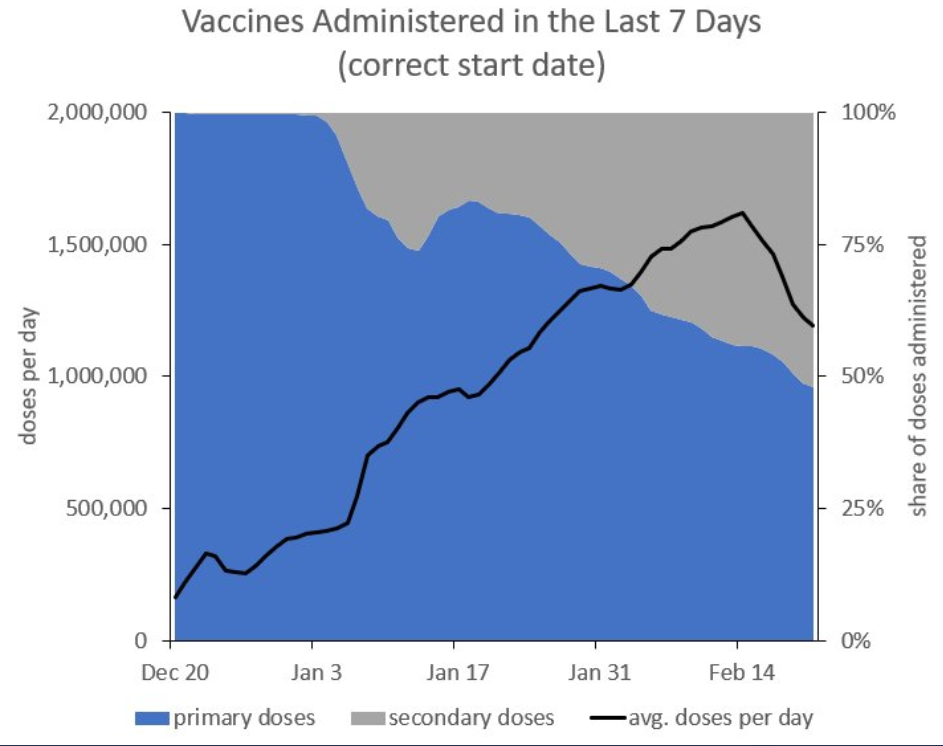
A Majority of Doses are Now Second Doses
As of Feb. 18 (last day of full data) we gave out 817,708 second doses and just 702,426 first doses. In other words, a majority of doses are now second doses. As Daniel Bier writes this means that we are boosting some people from ~85% to ~95% protected when we could be vaccinating more first timers and getting them from 0% protected to ~85% protected.
If we followed the British rule and delayed the booster to 12 weeks, we could immediately more than *double* the number of people going from 0% protected to to ~85% protected. More first-doses would be great for the newly protected and more people at ~85% protection would also reduce transmission so there would be fewer new infections and less threat to the non-vaccinated.
The opportunity cost of not delaying the booster is measured in lives lost.
The First Dose is Good
WSJ: The Covid-19 vaccine developed by Pfizer Inc. and BioNTech SE generates robust immunity after one dose and can be stored in ordinary freezers instead of at ultracold temperatures, according to new research and data released by the companies.
The findings provide strong arguments in favor of delaying the second dose of the two-shot vaccine, as the U.K. has done . They could also have substantial implications on vaccine policy and distribution around the world, simplifying the logistics of distributing the vaccine.
A single shot of the vaccine is 85% effective in preventing symptomatic disease 15 to 28 days after being administered, according to a peer-reviewed study conducted by the Israeli government-owned Sheba Medical Center and published in the Lancet medical journal. Pfizer and BioNTech recommend that a second dose is administered 21 days after the first.
The finding is a vindication of the approach taken by the U.K. government to delay a second dose by up to 12 weeks so it could use limited supplies to deliver a single dose to more people, and could encourage others to follow suit. Almost one-third of the U.K.’s adult population has now received at least one vaccine shot. Other authorities in parts of Canada and Europe have prioritized an initial shot, hoping they will have enough doses for a booster when needed.
Preliminary data also suggest that the other widely used vaccine in the U.K. developed by AstraZeneca PLC and the University of Oxford could have a substantial effect after a first dose .
The Israeli findings came from the first real-world data about the effect of the vaccine gathered outside of clinical trials in one of the leading nations in immunization against the coronavirus. Israel has given the first shot to 4.2 million people—more than two-thirds of eligible citizens over 16 years old—and a second shot to 2.8 million, according to its health ministry. The country has around 9.3 million citizens.
…”This groundbreaking research supports the British government’s decision to begin inoculating its citizens with a single dose of the vaccine,” said Arnon Afek, Sheba’s deputy director general.
The Israeli study is here. Data from Quebec also show that a single dose is highly effective, 80% or higher (Figure 3) in real world settings.
It’s becoming clearer that delaying the second dose is the right strategy but it was the right strategy back in December when I first started advocating for First Doses First. Waiting for more data isn’t “science,” it’s sometimes an excuse for an unscientific status-quo bias.
Approximately 16 million second doses have been administered in the US. If those doses had been first doses an additional 16 million people would have been protected from dying. Corey White estimates that every 4000 flu vaccinations saves a life which implies 4000 lives would have been saved by going to FDF. COVID, of course, is much deadlier than the flu–ten times as deadly or more going by national death figures (so including transmission and case fatality rate)– so 40,000 deaths is back of the envelope. Let’s do some more back-of-the-envelope calculations. Since Dec. 14, there have been approximately 10 million confirmed cases in the United States and 200,000 deaths. There are 200 million adults in the US so 1/1000 adults has died from COVID, just since Dec. 14. If we use 1/1000 as the risks of a random adult dying from COVID, then an additional 16 million vaccinations would have saved 16,000 lives. But that too is likely to be an underestimate since the people being vaccinated are not a random sample of adults but rather adults with a much higher risk of dying from COVID. Two to four times that number would not be unreasonable so an additional 16 million vaccinations might have avoided 32,000-64,000 deaths. Moreover, an additional 16 million first doses would have reduced transmission. None of these calculations is very good but they give a ballpark.
It is excellent news that the vaccine is stable for some time using ordinary refrigeration. Scott Duke Kominers and I argue that there is lot of unused vaccination capacity at the pharmacies and reducing the cold storage requirement will help to bring that unused capacity online. The announcement is also important for a less well understood reason. If Pfizer is only now learning that ultra-cold storage isn’t necessary then we should be looking much more closely at fractional dosing.
When I said that we should delay the second dose, people would respond with “but the companies say 21 days and 28 days! Listen to the science!”. That’s not scientific thinking but magical thinking. Listening to the science was understanding that the clinical trial regimen was designed at speed with the sole purpose of getting the vaccines approved. The clinical trial was not designed to discover the optimal regimen for public health. Don’t get me wrong. Pfizer and Moderna did the right thing! But it was wrong to think that the public health authorities could simply rely on “the science” as if it were written on stone. Even cold-storage wasn’t written on stone! Now that the public health authorities know that the clinical trial regimen isn’t written in stone they should be more willing to consider policies such as delaying the second dose and fractional dosing.
We are nearing the end in the US but delaying the second dose and other dose-stretching policies are going to be important in other countries.
Half Doses of Moderna Produce Neutralizing Antibodies
A new phase II study from Moderna shows that half-doses (50 μg) appear to be as good as full doses (100 ug) at generating correlates of protection such as neutralizing antibodies.
In this randomized, controlled phase 2 trial, the SARS-CoV-2 vaccine candidate mRNA-1273, administered as a two-dose vaccination regimen at 50 and 100 μg, exhibited robust immune responses and an acceptable safety profile in healthy adults aged 18 years and older. Local and systemic adverse reactions were mostly mild-to-moderate in severity, were ≤4 days of median duration and were less commonly reported in older compared with younger adults. Anti-SARS-CoV-2 spike binding and neutralizing antibodies were induced by both doses of mRNA-1273 within 28 days after the first vaccination, and rose substantially to peak titers by 14 days after the second vaccination, exceeding levels of convalescent sera from COVID-19 patients. The antibodies remained elevated through the last time point assessed at 57 days. Neutralizing responses met criteria for seroconversion within 28 days after the first vaccination in the majority of participants, with rates of 100% observed at 14 and 28 days after the second vaccination. While no formal statistical testing was done, binding and neutralizing antibody responses were generally comparable in participants who received the 100 μg mRNA-1273 and the 50 μg dose at all time points and across both age groups. Overall, the results of this randomized, placebo-controlled trial extend previous immunogenicity and safety results for mRNA-1273 in the phase 1 study in an expanded cohort including participants older than 55 years of age [16, 19].
[These data] confirm that a robust immune response is generated at both 50 and 100 ug dose levels.
As I wrote earlier, halving the dose is equivalent to instantly doubling the output of every Moderna factory.
See my piece in the Washington Post on getting to V-day sooner for an overview of dose stretching strategies.
Addendum: France says one dose is sufficient for previously COVID infected.
“Second Doses” post-mortem
The most striking thing about the Biden administration shift to a version of “First Doses First” is how little protest there has been. Given how many public health experts were upset about the idea only a few days ago, you might expect them to organize a Wall Street Journal petition from hundreds of their colleagues: “Biden administration proposal endangers the lives of millions of Americans.”
But of course they won’t do that. Some of that is pro-Democrat partisanship, but that is not even the main factor. One reason is that public health experts, with their medical and quasi-medical backgrounds, typically have very little sense of how to respond in the public arena if challenged. For instance, not a single one stepped forward with a calculation to defend “Second Doses.” They are not especially good at “the internet rules of the game,” which of course are now supreme (not always for the best, to be clear).
The second and probably most important reason is that, as I had explained, sins of omission are treated as far less significant than sins of commission. Now that a version of “First Doses First” is on the verge of becoming policy, to do nothing about that is only a sin of omission, and thus not so bad. Remarkable! Status quo bias really matters here.
I haven’t seen a single peep on Twitter opposing the new policy.
Just keep all this in mind the next time you see a debate over public health policy. There is often less behind the curtain than you might think.
Half-Doses as Good as Full?
NYTimes: A top official of Operation Warp Speed floated a new idea on Sunday for stretching the limited number of Covid-19 vaccine doses in the United States: Halving the dose of each shot of Moderna’s vaccine to potentially double the number of people who could receive it.
Data from Moderna’s clinical trials demonstrated that people between the ages of 18 and 55 who received two 50-microgram doses showed an “identical immune response” to the standard of two 100-microgram doses, said the official, Dr. Moncef Slaoui.
Dr. Slaoui said that Operation Warp Speed was in discussions with the Food and Drug Administration and the pharmaceutical company Moderna over implementing the half-dose regimen. Moderna did not respond immediately to a request for comment.
Each vaccine would still be delivered in two, on-schedule doses four weeks apart, Dr. Slaoui said in an interview with “CBS’s Face the Nation.” He said it would be up to the F.D.A. to decide whether to move forward with the plan.
Half dosing would double Moderna doses permanently rather than temporarily (as with First Doses First). Thus, I would be very happy to see half-dosing and it would obviate the need for FDF.
I and a handful of others started to discuss and advocate First Doses First on Dec. 8 and many times since then. The advocacy was then joined by Tony Blair and by many epidemiologists, immunologists, vaccine researchers, physicians and public health experts as well, of course, by the British experts on the Joint Committee on Vaccination and Immunisation. It’s clear that the FDA and Operation Warp Speed are now feeling the pressure to take some serious actions to increase supply. If so, my small efforts will have had a very high return.
Keep the pressure on.
Addendum: By the way, the British have yet to approve the Moderna vaccine (probably because they can’t get doses for some time anyway) and the AstraZeneca vaccine appears to work better with a longer dosing interval. So FDF makes sense for the British and we can do half-dosing on Moderna, potentially setting a new and beneficial standard for the entire world.
First Dose First
Writing on twitter Keith Klugman, world expert on infectious diseases and director of the Gates Foundation Pneumonia program, supports a policy I have argued for–First Doses First.
First doses of Pfizer/Moderna vaccines are 90%+ effective after 14 days. Most high risk lives will be saved by giving all these limited early supplies of vaccine as first doses – second doses can be given later if first dose effectiveness wanes or when supply improves
Here’s a way of thinking about this policy. Suppose you are scheduled for your second dose of the Pfizer or Moderna vaccine but you have the option of giving your second dose to your spouse as their first dose. Would you?
If the answer is yes then can you ethically deny this to someone else’s spouse?
Keep in mind that we have at least three more vaccines that could be available in as little as 12 weeks, Astra-Zeneca, Johnson & Jonson and Novavax. We are also pushing for more doses from Pfizer and we should be willing to pay top-dollar for those doses. As those vaccines come online we can deliver second doses.
Addendum: If you are 75 and your spouse is 25 then maybe you wouldn’t give your second dose to your spouse and that too ought to help us think about the larger questions of allocation.
The case for geographically concentrated vaccine doses
Here goes:
A central yet neglected point is that vaccines should not be sent to each and every part of the U.S. Instead, it would be better to concentrate distribution in a small number of places where the vaccines can have a greater impact.
Say, for the purposes of argument, that you had 20,000 vaccine doses to distribute. There are about 20,000 cities and towns in America. Would you send one dose to each location? That might sound fair, but such a distribution would limit the overall effect. Many of those 20,000 recipients would be safer, but your plan would not meaningfully reduce community transmission in any of those places, nor would it allow any public events to restart or schools to reopen.
Alternatively, say you chose one town or well-defined area and distributed all 20,000 doses there. Not only would you protect 20,000 people with the vaccine, but the surrounding area would be much safer, too. Children could go to school, for instance, knowing that most of the other people in the building had been vaccinated. Shopping and dining would boom as well.
Here is one qualifier, but in fact it pushes one further along the road to geographic concentration:
Over time, mobility, migration and mixing would undo some of the initial benefits of the geographically concentrated dose of vaccines. That’s why the second round of vaccine distribution should go exactly to those people who are most likely to mix with the first targeted area. This plan reaps two benefits: protecting the people in the newly chosen second area, and limiting the ability of those people to disrupt the benefits already gained in the first area.
In other words, if the first doses went (to choose a random example) to Wilmington, Delaware, the next batch of doses should go to the suburbs of Wilmington. In economics language [behind this link is a highly useful Michael Kremer paper], one can say that Covid-19 infections (and protections) have externalities, and there are increasing returns to those externalities. That implies a geographically concentrated approach to vaccine distribution, whether at the federal or state level.
Here is another qualifier:
…there will be practical limits on a fully concentrated geographic distribution of vaccines. Too many vaccines sent to too few places will result in long waits and trouble with storage. Nonetheless, at the margin the U.S. should still consider a more geographically concentrated distribution than what it is likely to do.
Do you think that travel restrictions have stopped the spread of the coronavirus? (Doesn’t mean you have to favor them, all things considered.) Probably yes. If so, you probably ought to favor a geographically concentrated initial distribution of the vaccine as well — can you see why it is the same logic? Just imagine it spreading out like stones on a Go board.
Of course we are not likely to do any of this. Here is my full Bloomberg column.
Extreme carcinogenic doses for rats
Here is a defense of using those rat tests to judge what will cause cancer in humans:
The "junk science" they are referring to is the long-standing and
well-confirmed practice of identifying chemicals likely to cause cancer
in humans by testing them in animals. The animals (rodents) are a
standard model for biological processes of relevance to humans (which
is why drug companies and medical researchers have been using them for
a century). They are well understood and are the only sentinels for
detecting carcinogenicity of any use to public health. Since chemically
induced cancer has a latency period of decades (typically 20 years or
more), waiting for it to appear in human populations would meant that
once detected, even if exposure would cease instantly (which can never
happen), it would take another 20 or more years to eliminate the
cancers from exposure (all the cancers induced in the 20 years exposure
prior to detection). But even then, the chances of detecting any but
the most powerful carcinogens in human populations (via epidemiology)
is small. Epidemiology is a very insensitive tool. I say this with some
authority, as I am a cancer epidemiologist specializing in chemical
exposures and have authored numerous peer reviewed studies in that area
over many years.
The main rhetorical lever ACSH employs is the
use of high doses in the animal studies, doses that are much higher
than usually faced by humans. But as ACSH knows well (but didn’t
divulge) there is a technical requirement for using these doses. If one
were to use doses in animals predicted to cause cancer at a rate we
would consider a public health hazard, we would need tens of thousands
of animals to test a single dose, mode of exposure and rodent species
or strain. This makes using those doses infeasible. Thus a Maximum
Tolerated Dose is used, one that causes no other pathology except
possibly cancer and doesn’t result in more than a 10% weight loss. The
assumption here is that something that causes cancer at high doses in
these animals will also do so at low doses. This is biologically
reasonable. It is a (surprising) fact, that most chemicals, given in no
matter how high a dose, won’t cause the very unusual and specific
biological effect of turning an animal cell cancerous. Cancer cells are
not "damaged" cells in the individual sense but "super cells," capable
of out competing normal cells. It is only in the context of the whole
organism that there is a problem. It is not surprising, then, that very
few chemicals would have be ability to turn a normal cell into a
biological super cell of this type. Estimates are that is far less than
10%, perhaps only 1% of all chemicals that have this ability. Thus
western industrial civilization doesn’t have to come to a screeching
halt if we eliminate industrial chemical carcinogens from our
environment.We know of no false negatives with this process.
Every chemical we know that causes cancer in humans also does so in
rodents (with the possible exception of inorganic trivalent arsenic,
which is equivocal).
Here is the full post. I’m not close to having the expertise to evaluate these claims, but two points. First, the author is highly qualified; as a blogger he is anonymous but I can vouch for his credentials. Second, it should be the self-appointed task of bloggers to pass along arguments which either struck them or which might shake up their readers.
Does Britain Have High or Low State Capacity?
Tim Harford writing at the FT covers the question “Is it even possible to prepare for a pandemic?” drawing on my paper with Tucker Omberg.
[I]n an unsettling study published late last year, the economists Robert Tucker Omberg and Alex Tabarrok took a more sophisticated look at this question and found that “almost no form of pandemic preparedness helped to ameliorate or shorten the pandemic”. This was true whether one looked at indicators of medical preparedness, or softer cultural factors such as levels of individualism or trust. Some countries responded much more effectively than others, of course — but there was no foretelling which ones would rise to the challenge by looking at indicators published in 2019. One response to this counter-intuitive finding is that the GHS Index doesn’t do a good job of measuring preparedness. Yet it seemed plausible at the time and it still looks reasonable now.
…perhaps we need to take the Omberg/Tabarrok study seriously: maybe conventional preparations really won’t help much. What follows? One conclusion is that we should prepare, but in a different way….Preparing a nimble system of testing and of compensating self-isolating people would not have figured in many 2019 pandemic plans. It will now. Another form of preparation which might yet pay off is sewage monitoring, which can cost-effectively spot the resurgence of old pathogens and the appearance of new ones, and may give enough warning to stop some future pandemics before they start. And, says Tabarrok, “Vaccines, vaccines, vaccines”. The faster our systems for making, testing and producing vaccines, the better our chances; all these things can be prepared.
One thing that did seem to matter, as Tim notes, was state capacity. In other words, it’s not so much being prepared as being prepared to act. And here I have a mild disagreement with Tim. He writes:
In an ill-prepared world, the UK is often thought to have been more ill-prepared than most, perhaps because of the strains caused by austerity and the distractions of the Brexit process.
My view is that the UK got three very important things right. The UK was the first stringent authority to approve a COVID vaccine. The UK switched to first doses first and the UK produced and ran the most important therapeutics trial, the Recovery trial. Each of these decisions and programs saved the lives of tens of thousands of Britons. The Recovery trial may have saved millions of lives worldwide.
I don’t claim that Britain did everything right, or that they did all that they could have done, but these three decisions were important, bold and correct. The coexistence of both high and low state capacity within the same nation can be surprising. The United States, for example, achieved an impressive feat with Operation Warp Speed, yet simultaneously, the Centers for Disease Control and Prevention (CDC) flailed and failed. Likewise, India maintains a commendable space program and an efficient electoral system, even while struggling with tasks that seem comparatively simpler, like issuing driver’s licenses.
Instead of painting countries with a broad brush of ‘high’ or ‘low’ state capacity, we should recognize multi-dimensionality and divergence. How do political will, resources, institutional robustness, culture, and history explain capacity divergence? If we understood the reasons for capacity divergence we might be able to improve state capacity more generally. Or we might better be able to assign tasks to state or market with perhaps very different assignments depending on the country.
Who was really for “focused protection of the vulnerable”?
Yes I do mean during the Covid-19 epidemic. As a follow-up post to Alex’s, and his follow-up, here are some of the effective measures in protecting the vulnerable, or they would have been more effective, had we done them better:
1. Vaccines, including speedy approval of same.
2. Prepping hospitals in January, once it became clear we should be doing so. That also would have limited lockdowns! And yet we did basically nothing.
3. Speeding up and improving the research process for anti-Covid remedies and protections.
4. First Doses First, when that policy was appropriate, among other policy ideas (NYT).
5. Effective and rapid testing equipment, readily available on the market.
If you were out promoting those ideas, you were acting in favor of protecting the vulnerable. If you were not out promoting those ideas, but instead talked about “protecting the vulnerable” in a highly abstract manner, you were not doing much to protect the vulnerable.
And here are three actions that endangered the vulnerable rather than protecting them:
5. Publishing papers suggesting a very, very low Covid-19 mortality rate, and then sticking with those results in media appearances after said results appeared extremely unlikely to be true.
6. Maintaining vague (or in some cases not so vague) affiliations with anti-vax groups.
7. Not having thought through how “herd immunity” doctrines might be modified by ongoing mutations.
Keep all that in mind the next time you hear the phrase “protecting the vulnerable.”
Dose Stretching for the Monkeypox Vaccine
We are making all the same errors with monkeypox policy that we made with Covid but we are correcting the errors more rapidly. (It remains to be seen whether we are correcting rapidly enough.) I’ve already mentioned the rapid movement of some organizations to first doses first for the monkeypox vaccine. Another example is dose stretching. I argued on the basis of immunological evidence that A Half Dose of Moderna is More Effective Than a Full Dose of AstraZeneca and with Witold Wiecek, Michael Kremer, Chris Snyder and others wrote a paper simulating the effect of dose stretching for COVID in an SIER model. We even worked with a number of groups to accelerate clinical trials on dose stretching. Yet, the idea was slow to take off. On the other hand, the NIH has already announced a dose stretching trial for monkeypox.
Scientists at the National Institutes of Health are getting ready to explore a possible work-around. They are putting the finishing touches on the design of a clinical trial to assess two methods of stretching available doses of Jynneos, the only vaccine in the United States approved for vaccination against monkeypox.
They plan to test whether fractional dosing — using one-fifth of the regular amount of vaccine per person — would provide as much protection as the current regimen of two full doses of the vaccine given 28 days apart. They will also test whether using a single dose might be enough to protect against infection.
The first approach would allow roughly five times as many people to be vaccinated as the current licensed approach, and the latter would mean twice as many people could be vaccinated with existing vaccine supplies.
…The answers the study will generate, hopefully by late November or early December, could significantly aid efforts to bring this unprecedented monkeypox outbreak under control.
Another interesting aspect of the dose stretching protocol is that the vaccine will be applied to the skin, i.e. intradermally, which is known to often create a stronger immune response. Again, the idea isn’t new, I mentioned it in passing a couple of times on MR. But we just weren’t prepared to take these step for COVID. Nevertheless, COVID got these ideas into the public square and now that the pump has been primed we appear to be moving more rapidly on monkeypox.
Addendum: Jonathan Nankivell asked on the prediction market, Manifold Markets, ‘whether a 1/5 dose of the monkey pox vaccine would provide at least 50% the protection of the full dose?’ which is now running at a 67% chance. Well worth doing the clinical trial! Especially if we think that the supply of the vaccine will not expand soon.
Status Quo Bias
Here is the lead sentence from a CNN piece on vaccine boosters:
Even though the biopharmaceutical company Pfizer has announced that it might be time to consider giving a third dose of its coronavirus vaccine to people, many doctors and public health officials argue that it’s more beneficial to get shots into the arms of the unvaccinated right now than to boost those who are already fully vaccinated.
Delaying the 3rd dose to get out more 2nd doses is a perfectly reasonable position. What’s interesting is that today delaying the 3rd dose is conventional wisdom and yet this is exactly the same argument that I made for delaying the 2nd dose, i.e. first doses first (FDF), back in December of 2020. At that time, however, the argument was controversial. My point, isn’t that FDF has won the argument. My point is that what we are seeing, then and now, is status quo bias.
In December, status quo bias meant that people wanted to find a reason to stick with the status quo, i.e. 2 doses, and so they argued that delaying the second dose was “risky.” Today, people still want to stick with the status quo and so they argue that third doses are “risky”, i.e. delaying the third dose is now the less risky idea. The argument–it’s smart to protect more people with fewer doses–hasn’t changed but, without even realizing it, people are now making the argument that they once denied.
The logic hasn’t convinced people but previously the logic opposed the status quo and now it supports the status quo so what was once denied is now accepted. What was in December the riskier choice now becomes the safer choice. With motivated reasoning, when the motivation changes so does the reasoning.
Hat tip: Iamamish
Addendum: People will respond in the comments, but actually the situations are different. Indeed, things are different and the situation has changed. But that’s not the only or even the primary driver. If we had started with a 3-dose regimen, delaying the third dose would have seemed just as “risky” as delaying the second dose in a 2-dose regimen.
Addendum 2: The WHO today called for a moratorium on boosters until more countries are vaccinated.