Results for “rapid test” 292 found
The FDA’s Lab-Test Power Grab
The FDA is trying to gain authority over laboratory developed tests (LDTs). It’s a bad idea. Writing in the WSJ, Brian Harrison, who served as chief of staff at the U.S. Department of Health and Human Services, 2019-2021 and Bob Charrow, who served as HHS general counsel, 2018-2021, write:
We both were involved in preparing the federal Covid-19 public-health emergency declaration. When it was signed on Jan. 31, 2020, the intent was to cut red tape and maximize regulatory flexibility to allow a nimble response to an emerging pandemic.
Unknown to us, the next day the FDA went in the opposite direction: It issued a new requirement that labs stop testing for Covid-19 and first apply for FDA authorization. At that time, LDTs were the only Covid tests the U.S. had, and many were available and ready to be used in labs around the country. But since the process for emergency-use authorization was extremely burdensome and slow—and because, as we and others in department leadership learned, it couldn’t process applications quickly—many labs stopped trying to win authorization, and some pleaded for regulatory relief so they could test.
Through this new requirement the FDA effectively outlawed all Covid-19 testing for the first month of the pandemic when detection was most critical. One test got through—the one developed by the Centers for Disease Control and Prevention—but it proved to be one of the highest-profile testing failures in history because the entire nation was relying on the test to work as designed, and it didn’t.
When we became aware of the FDA’s action, one of us (Mr. Harrison) demanded an immediate review of the agency’s legal authority to regulate these tests, and the other (Mr. Charrow) conducted the review. Based on the assessment, a determination was made by department leadership that the FDA shouldn’t be regulating LDTs.
Congress has never expressly given the FDA authority to regulate the tests. Further, in 1992 the secretary of health and human services issued a regulation stating that these tests fell under the jurisdiction of the Centers for Medicare and Medicaid Services, not the FDA. Bureaucrats at the FDA have tried to ignore this rule even though the Supreme Court in Berkovitz v. U.S. (1988) specifically admonished the agency for ignoring federal regulations.
Loyal readers will recall that I covered this issue earlier in Clement and Tribe Predicted the FDA Catastrophe. Clement, the former US Solicitor General under George W. Bush and Tribe, a leading liberal constitutional lawyer, rejected the FDA claims of regulatory authority over laboratory developed tests on historical, statutory, and legal grounds but they also argued that letting the FDA regulate laboratory tests was a dangerous idea. In a remarkably prescient passage, Clement and Tribe (2015, p. 18) warned:
The FDA approval process is protracted and not designed for the rapid clearance of tests. Many clinical laboratories track world trends regarding infectious diseases ranging from SARS to H1N1 and Avian Influenza. In these fast-moving, life-or-death situations, awaiting the development of manufactured test kits and the completion of FDA’s clearance procedures could entail potentially catastrophic delays, with disastrous consequences for patient care.
Clement and Tribe nailed it. Catastrophic delays, with disastrous consequences for patient care is exactly what happened. Thus, Harrison and Charrow are correct, giving the FDA power over laboratory derived tests has had and will have significant costs.
Testing Freedom
I did a podcast with Brink Lindsey of the Niskanen Center. Here’s one bit on the FDA’s long-history of banning home tests:
Brink Lindsey: …it’s on the rapid testing that we had inexplicable delays. Rapid tests, home tests were ubiquitous in Europe and Asia months before they were in the United States. What was going on?
Alex Tabarrok: So I think it’s not actually inexplicable because the FDA has a long, long history of just hating people testing themselves. So the FDA was against pregnancy tests, they didn’t like that, they said women they need to consult with a doctor, only the physician can do the test because literally women could become hysterical if they were pregnant or if they weren’t pregnant, this was a safety issue. There was no question that the test itself was safe or worked. Instead what the FDA said, “We can regulate this because the user using it, this could create safety issues because they could commit suicide or they could do something crazy.” So they totally expanded the meaning of safety from is the test safe to can somebody be trusted to use a pregnancy test?
Then we had exactly the same thing with AIDS testing. So we delayed personal at-home tests for AIDS for literally 25 years. 25 years these tests were unavailable because the FDA again said, “Well, they’re dangerous.” And why are they dangerous? “Well, we don’t know what people will do with this knowledge about their own bodies.” Now, of course, you can get an HIV test from Amazon and the world hasn’t collapsed. They did the same thing with genetic tests from companies like 23andMe. So I said, “Our bodies ourselves, our DNA ourselves.” That people have a right to know about the functioning of their own bodies. This to me is a very clear violation of the Constitutions on multiple respects. It just stuns me, it just stuns me that anybody could think that you don’t have a right to know, we’re going to prevent you from learning something about the operation of your own body.
Again, the issue here was never does the test work. In fact, the labs which produce these tests, those labs are regulated outside of the FDA. So whether the test actually works, whether yes, it identifies this gene, all issues of that nature, what is the sensitivity and the specificity, are the tests produced in a proper laboratory, I don’t have a lot of problem with that because that’s all something which the consumers themselves would want. What I do have a problem with is then the FDA saying, “No, you can’t have access to this test because we don’t know what you’re going to do about it, what you’re going to think about it.” And that to me is outrageous.
Here’s the full transcript and video.
Two brutal tests — can you pass them?
We all give people “tests” when we meet them, whether we are consciously aware of it or not. Here are two of mine:
1. The chess test. When I played chess in my youth, I would commonly analyze games with other players. You would then rapidly learn just how much and how quickly the other player could figure out the position and see imaginative variations. Some players maybe had equal or even inferior results to mine (I had a good work ethic and took no drugs), but it was obvious they were greater talents at analysis. Top chess players who worked with Bobby Fischer also attest that in this regard he was tops, not just “another great player.” That was true even before he was good enough and steady enough to become world champion.
When talking ideas with people, the same issue surfaces — just how quickly and how imaginatively do they grasp what is going on? You should put aside whatever they have or have not accomplished. How much do they have this Bobby Fischer-like capacity to analyze? No matter what their recent results have been (remember how Efim Geller used to kick Fischer’s butt in actual games?).
2. The art test. Take a person’s favorite genres of art, music, whatever. But something outside of their work lives. Maybe it can even be sports. How deeply do they understand the said subject matter? At what kind of level can they talk about it or enjoy it or maybe even practice it?
Remember in Hamlet, how Hamlet puts on a play right before the King’s eyes, to see how the King reacts to “art”?
Here we are testing for sensibility more than any kind of rigorous analysis, though the analysis test may kick in as well. Just how deep is the person’s deepest sensibility?
If you are investing in talent, you probably would prefer someone really good at one of these tests over someone who is “pretty good” at both of them.
3. All other tests.
Now, people can be very successful while failing both “the chess test” and “the art test.” In fact, most successful people fail both of these tests. Still, their kinds of success will be circumscribed. They are more likely to be hard-working, super-sharp, and accomplished, perhaps charismatic as well, while lacking depth and imaginative faculty in their work.
Nonetheless they will be super-focused on being successful.
I call this the success test.
Now if someone can pass the chess test, the art test, and the success test with flying colors…there are such people!
And if the person doesn’t pass any of those tests, they still might be just fine, but there will be a definite upper cap on their performance.
Why Doesn’t the United States Have Test Abundance?!
We have vaccine abundance in the United States but not test abundance. Germany has test abundance. Tests are easily available at the supermarket or the corner store and they are cheap, five tests for 3.75 euro or less than a dollar each. Billiger! In Great Britain you can get a 14 pack for free. The Canadians are also distributing packs of tests to small businesses for free to test their employees.
In the United States, the FDA has approved less than a handful of true at-home tests and, partially as a result, they are expensive at $10 to $20 per test, i.e. more than ten times as expensive as in Germany. Germany has approved over 50 of these tests including tests from American firms not approved in the United States. The rapid tests are excellent for identifying infectiousness and they are an important weapon, alongside vaccines, for controlling viral spread and making gatherings safe but you can’t expect people to use them more than a handful of times at $10 per use.
We ought to have testing abundance in the US and not lag behind Germany, the UK and Canada. As usual, I say if it’s good enough for the Germans it’s good enough for me.
Addendum: The excellent Michael Mina continues to bang the drum.
My Congressional Testimony
I thought the meeting went well. I made four points.
- It is not too late to do more.
- We should invest in nasal and oral vaccines.
- We should vaccinate the world.
- We should stretch doses through fractional dosing and delaying the second dose, this will be important to vaccinate the world quickly.
One observation. Lots of people are talking about vaccine hesitancy but I am one of the few people who have been talking about nasal and oral vaccines which are the only really solid approach to the issue that I have seen.
My best line:
The unvaccinated are the biggest risk for generating mutations and new variants. You have heard of the South Africa and Brazilian variants, well the best way to protect your constituents from these and other variants is to vaccinate South Africans and Brazilians.
I also got in the last word in Q&A when discussing the pause of J&J:
For the rest of the world it is important to underline that it is most important to get vaccinated now. Use the AstraZeneca vaccine, use the Johnnson & Johnson vaccine…don’t wait for Moderna or Pfizer, it is going to take too long…start your vaccination program early…vaccinate as quickly as possible, that is the route to health and wealth.
See Western Warnings Tarnish Vaccines the World Badly Needs for the beginnings of a disaster. Note that if J&J and AZ are tarnished or knocked out of the vaccine arsenal then dose stretching and investing in more capacity are going to be even more important.
I also submitted five excellent and important pieces to Congress:
Canadian statement on delaying the second dose.
National Advisory Committee on Immunization (NACI) Canada. 2021. “COVID-19 Vaccine Extended Dose Interval for Canadians: NACI Recommendation.” Government of Canada. March 3, 2021. https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/rapid-response-extended-dose-intervals-covid-19-vaccines-early-rollout-population-protection.html.
Value of vaccine capacity and additional investments.
Castillo, Juan Camilo, Amrita Ahuja, Susan Athey, Arthur Baker, Eric Budish, Tasneem Chipty, Rachel Glennerster, et al. 2021. “Market Design to Accelerate COVID-19 Vaccine Supply.” Science, February. https://doi.org/10.1126/science.abg0889.
Efficacy of the first dose from NEJM.
Skowronski, Danuta, and Gaston Serres De. 2021. “Letter to the Editor on Safety and Efficacy of the BNT162b2 MRNA Covid-19 Vaccine.” New England Journal of Medicine, February 17, 2021. https://doi.org/10.1056/NEJMc2036242.
Overview of dose stretching policies (with links in the online version).
Tabarrok, Alex. 2021. “What Are We Waiting For?” Washington Post, February 12, 2021, sec. Outlook. https://www.washingtonpost.com/outlook/2021/02/12/first-doses-vaccine-rules-fda/
A plan to vaccinate the world.
Agarwal, Ruchir, and Tristan Reed. 2021. “How to End the COVID-19 Pandemic by March 2022” SSRN. 2021. https://documents.worldbank.org/en/publication/documents-reports/documentdetail/181611618494084337/how-to-end-the-covid-19-pandemic-by-march-2022
Testing and the NFL
NYTimes: The Centers for Disease Control and Prevention and the National Institutes of Health announced a new initiative on Wednesday to help determine whether frequent, widespread use of rapid coronavirus tests slows the spread of the virus.
The program will make rapid at-home antigen tests freely available to every resident of two communities, Pitt County, N.C., and Hamilton County, Tenn., enough for a total of 160,000 people to test themselves for the coronavirus three times a week for a month.
“This effort is precisely what I and others have been calling for nearly a year — widespread, accessible rapid tests to help curb transmission,” said Michael Mina, an epidemiologist at Harvard University who has been a vocal proponent of rapid, at-home testing programs.
I guess this is good news it just feels like something that in a different time line, happened long ago. Here is Derek Thompson in an excellent piece making exactly that point:
Imagine a parallel universe where Americans were tested massively, constantly, without care for cost, while those who tested negative continued more or less about their daily life.
In fact, that parallel universe exists. It’s the National Football League.
..After an October outbreak, the NFL moved to daily testing of all its players and instituted new restrictions on player behavior and stricter rules on ventilation and social distancing. The league also used electronic tracking bracelets to trace close contacts of people who tested positive. Throughout the season, the NFL spent about $100 million on more than 900,000 tests performed on more than 11,000 players and staff members. In January, the CDC published an analysis of the league that concluded, “Daily testing allowed early, albeit not immediate, identification of infection,” enabling the league to play the game safely.
You could write off the NFL’s season as the idiosyncratic achievement of a greedy sport with nearly unlimited resources. But I can think of another self-interested institution with nearly unlimited resources: It’s the government of a country with a $20 trillion economy and full control over its own currency. Unlike the NFL, though, the U.S. never made mass testing its institutional priority.
“The NFL was almost like a Korea within the United States,” Alex Tabarrok told me. “And it’s not just the NFL. Many universities have done a fabulous job, like Cornell. They have followed the Korea example, which is repeated testing of students combined with quick isolation in campus dorms. Mass testing is a policy that works in practice, and it works in theory. It’s crazy to me that we didn’t try it.” Tabarrok said we can’t be sure that a Korean or NFL-style approach to national testing would have guaranteed Korean or NFL-style outcomes. After all, that would have meant averting about 500,000 deaths. Rather, he said, comprehensive early testing was our best shot at reducing deaths and getting back to normal faster.
New CRISPR-based COVID-19 test uses smartphone cameras to spot virus RNA
This one brings us closer to the Star Trek medical universe:
Scientists at UC Berkeley and Gladstone Institutes have developed a new CRISPR-based COVID-19 diagnostic test that, with the help of a smartphone camera, can provide a positive or negative result in 15 to 30 minutes. Unlike many other tests that are available, this test also gives an estimate of viral load, or the number of virus particles in a sample, which can help doctors monitor the progression of a COVID-19 infection and estimate how contagious a patient might be.
“Monitoring the course of a patient’s infection could help health care professionals estimate the stage of infection and predict, in real time, how long is likely needed for recovery and how long the individual should quarantine,” said Daniel Fletcher, a professor of bioengineering at Berkeley and one of the leaders of the study…
The new diagnostic test takes advantage of the CRISPR Cas13 protein, which directly binds and cleaves RNA segments. This eliminates the DNA conversion and amplification steps and greatly reduces the time needed to complete the analysis.
“One reason we’re excited about CRISPR-based diagnostics is the potential for quick, accurate results at the point of need,” [Jennifer] Doudna said. “This is especially helpful in places with limited access to testing or when frequent, rapid testing is needed. It could eliminate a lot of the bottlenecks we’ve seen with COVID-19.”
In the test, CRISPR Cas13 proteins are “programmed” to recognize segments of SARS-CoV-2 viral RNA and then combined with a probe that becomes fluorescent when cleaved. When the Cas13 proteins are activated by the viral RNA, they start to cleave the fluorescent probe. With the help of a handheld device, the resulting fluorescence can be measured by the smartphone camera. The rate at which the fluorescence becomes brighter is related to the number of virus particles in the sample.
And:
Now that the CRISPR-based assay has been developed for SARS-CoV-2, it could be modified to detect RNA segments of other viral diseases, like the common cold, influenza or even human immunodeficiency virus. The team is currently working to package the test into a device that could be made available at clinics and other point-of-care settings and that one day could even be used in the home.
“The eventual goal is to have a personal device, like a mobile phone, that is able to detect a range of different viral infections and quickly determine whether you have a common cold or SARS-Cov-2 or influenza,” Fletcher said. “That possibility now exists, and further collaboration between engineers, biologists and clinicians is needed to make that a reality.”
I recall once asking Silvana Konermann: “What am I going to buy at the CRISPR store?” Well, this is what you are going to buy at the CRISPR store.
Here is the article. And funded by Fast Grants, I am happy to say. Quite the week for science, yes?
Toward a universal medical test?
UC San Francisco scientists have developed a single clinical laboratory test capable of zeroing in on the microbial miscreant afflicting a patient in as little as six hours – irrespective of what body fluid is sampled, the type or species of infectious agent, or whether physicians start out with any clue as to what the culprit may be.
The test will be a lifesaver, speeding appropriate drug treatment for the seriously ill, and should transform the way infectious diseases are diagnosed, said the authors of the study, published Nov. 9 in Nature Medicine.
The advance here is that we can detect any infection from any body fluid, without special handling or processing for each distinct body fluid,” said study corresponding author Charles Chiu, MD, PhD, a professor in the UCSF Department of Laboratory Medicine and director of the UCSF-Abbott Viral Diagnostics and Discovery Center.
Here is the full story, via David Lim.
The greatest gaming performance ever?
Or is chess a sport?
First Magnus Carlsen “privatizes” chess competition, naming the major tournament after himself, setting all of the rules, and becoming the residual claimant on the income stream.
He reshapes the entire format into a seven set, four months-long series of shorter tournaments, consisting of multiple games per day, 15 minutes per player per game, with increment. It seems most chess fans find this new format far more exciting and watchable than the last two world championship matches, which have featured 22 slow draws and only two decisive games (with the title decided by rapid tiebreakers in each case — why not just head to the rapids?).
Magnus won all but one of the “sets” or mini-tournaments, along the way regularly dispatching the game’s top players at an astonishing pace, often tossing them aside like mere rag dolls. Even the #2 and #3 rated players — Caruana and Ding Liren — stood little chance against his onslaught. Carlsen kept on winning these mini-tournaments against fields of ten players, typically all at a world class level.
A Final Four then led to a 38-game, seven-day showdown between Carlsen and Hikaru Nakamura, not decided until the very last set of moves yesterday. Note that at the more rapid pace Nakamura may well be a better player than Carlsen and is perhaps the only real challenge to him (at slower classical speeds Nakamura would be in the top twenty but is not at the very top of the rankings).
Nonetheless Carlsen prevailed. Nakamura had the upper hand in terms of initiative, but in the final five-minute tie-breaking round, Carlsen needed to pull out 1.5 of the last 2 points, which indeed he did. He drew by constructing an impregnable fortress against Nakamura’s Queen, and in the final “sudden death Armageddon” round a draw is equivalent to a victory for Black.
Along the way, at the same time, Magnus participated in Fantasy Football, competing against millions, at times holding the #1 slot and finishing #11 in what is a very competitive and demanding endeavor.
The new quicker, cheaper, supply chain robust saliva test
The FDA has just approved a new and important Covid-19 test:
“Wide-spread testing is critical for our control efforts. We simplified the test so that it only costs a couple of dollars for reagents, and we expect that labs will only charge about $10 per sample. If cheap alternatives like SalivaDirect can be implemented across the country, we may finally get a handle on this pandemic, even before a vaccine,” said Grubaugh.
One of the team’s goals was to eliminate the expensive saliva collection tubes that other companies use to preserve the virus for detection. In a separate study led by Wyllie and the team at the Yale School of Public Health, and recently published on medRxiv, they found that SARS-CoV-2 is stable in saliva for prolonged periods at warm temperatures, and that preservatives or specialized tubes are not necessary for collection of saliva.
Of course this part warmed my heart (doubly):
The related research was funded by the NBA, National Basketball Players Association, and a Fast Grant from the Emergent Ventures at the Mercatus Center, George Mason University.
The NBA had the wisdom to use its unique “bubble” to run multiple tests on players at once, to see how reliable the less-known tests would be. This WSJ article — “Experts say it could be key to increasing the nation’s testing capacity” — has the entire NBA back story. At an estimated $10 a pop, this could especially be a game-changer for poorer nations. Furthermore, it has the potential to make pooled testing much easier as well.
Here is an excerpt from the research pre-print:
The critical component of our approach is to use saliva instead of respiratory swabs, which enables non-invasive frequent sampling and reduces the need for trained healthcare professionals during collection. Furthermore, we simplified our diagnostic test by (1) not requiring nucleic acid preservatives at sample collection, (2) replacing nucleic acid extraction with a simple proteinase K and heat treatment step, and (3) testing specimens with a dualplex quantitative reverse transcription PCR (RT-qPCR) assay. We validated SalivaDirect with reagents and instruments from multiple vendors to minimize the risk for supply chain issues. Regardless of our tested combination of reagents and instruments from different vendors, we found that SalivaDirect is highly sensitive with a limit of detection of 6-12 SARS-CoV-2 copies/μL.
No need to worry and fuss about RNA extraction now. Here is the best simple explanation of the whole thing.
The researchers are not seeking to commercialize their advance, rather they are making it available for the general benefit of mankind. Here is Nathan Grubaugh on Twitter. Here is Anne Wyllie, also a Kiwi and a Kevin Garnett fan. A further implication of course is that the NBA bubble is not “just sports,” but also has boosted innovation by enabling data collection.
All good news of course, and Fast at that. And this:
“This could be one the first major game changers in fighting the pandemic,” tweeted Andy Slavitt, a former acting administrator of the Centers for Medicare and Medicaid Services in the Obama administration, who expects testing capacity to be expanded significantly. “Rarely am I this enthusiastic… They are turning testing from a bespoke suit to a low-cost commodity.”
And here is coverage from Zach Lowe. I am very pleased with the course of Fast Grants more generally, and you will be hearing more about it in the future.
Pooled Testing is Super-Beneficial
Tyler and I have been pushing pooled testing for months. The primary benefit of pooled testing is obvious. If 1% are infected and we test 100 people individually we need 100 tests. If we split the group into five pools of twenty then if we’re lucky, we only need five tests. Of course, chances are that there will be some positives in at least one group and taking this into account we will require 23.2 tests on average (5 + (1 – (1 – .01)^20)*20*5). Thus, pooled testing reduces the number of needed tests by a factor of 4. Or to put it the other way, under these assumptions, pooled testing increases our effective test capacity by a factor of 4. That’s a big gain and well understood.
An important new paper from Augenblick, Kolstad, Obermeyer and Wang shows that the benefits of pooled testing go well beyond this primary benefit. Pooled testing works best when the prevalence rate is low. If 10% are infected, for example, then it’s quite likely that all five pools will have at least one positive test and thus you will still need nearly 100 tests (92.8 expected). But the reverse is also true. The lower the prevalence rate the fewer tests are needed. But this means that pooled testing is highly complementary to frequent testing. If you test frequently then the prevalence rate must be low because the people who tested negative yesterday are very likely to test negative today. Thus from the logic given above, the expected number of tests falls as you tests more frequently (per test-cohort).
Suppose instead that people are tested ten times as frequently. Testing individually at this frequency requires ten times the number of tests, for 1000 total tests. It is therefore natural think that group testing also requires ten times the number of tests, for more than 200 total tests. However, this estimation ignores the fact that testing ten times as frequently reduces the probability of infection at the point of each test (conditional on not being positive at previous test) from 1% to only around .1%. This drop in prevalence reduces the number of expected tests – given groups of 20 – to 6.9 at each of the ten testing points, such that the total number is only 69. That is, testing people 10 times as frequently only requires slightly more than three times the number of tests. Or, put in a different way, there is a “quantity discount” of around 65% by increasing frequency.
Peter Frazier, Yujia Zhang and Massey Cashore also point out that you could also do an array-protocol in which each person is tested twice but in two different groups–this doubles the number of initial tests but limits the number of false-positives (both tests must be positive) and the number of needed retests. (See figure.). 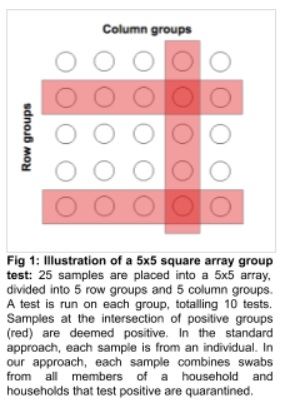
Moreover, we haven’t yet taken into account the point of testing which is to reduce the prevalence rate. If we test frequently we can reduce the prevalence rate by quickly isolating the infected population and by reducing the prevalence rate we reduce the number of needed tests. Indeed, under some parameters it’s possible to increase the frequency of testing and at the same time reduce the total number of tests!
We can do better yet if we group individuals whose risks are likely to be correlated. Consider an office building with five floors and 100 employees, 20 per floor. If the prevalence rate is 1% and we test people at random then we will need 23.2 tests on average, as before. But suppose that the virus is more likely to transmit to people who work on the same floor and now suppose that we pool each floor. Holding the total prevalence rate constant, we are now likely to have a zero prevalence rate on four floors and a 5% prevalence rate on one floor. We don’t know which floor but it doesn’t matter–the expected number of tests required now falls to 17.8.
The authors suggest using machine learning techniques to uncover correlations which is a good idea but much can be done simply by pooling families, co-workers, and so forth.
The government has failed miserably at controlling the pandemic. Tens of thousands of people have died who would have lived under a more competent government. The FDA only recently said they might allow pooled testing, if people ask nicely. Unbelievably, after telling us we don’t need masks (supposedly a noble lie to help limit shortages), the CDC is still disparaging testing of asymptomatic people (another noble lie?) which is absolutely disastrous. Paul Romer is correct, testing capacity won’t increase until we put soft drink money behind advance market commitments and start using techniques such as pooled testing. Fortunately or sadly, depending on how you look at it, it’s not too late to do better. Some universities are now proposing rapid, frequent testing using pooling. Harvard will test every three days. Cornell will test frequently. Delaware State will test weekly. Lets hope the idea spreads from the ivory tower.
FDA Allows Pooled Tests and a Call for Prizes
The FDA has announced they will no longer forbid pooled testing:
In order to preserve testing resources, many developers are interested in performing their testing using a technique of “pooling” samples. This technique allows a lab to mix several samples together in a “batch” or pooled sample and then test the pooled sample with a diagnostic test. For example, four samples may be tested together, using only the resources needed for a single test. If the pooled sample is negative, it can be deduced that all patients were negative. If the pooled sample comes back positive, then each sample needs to be tested individually to find out which was positive.
…Today, the FDA is taking another step forward by updating templates for test developers that outline the validation expectations for these testing options to help facilitate the preparation, submission, and authorization under an Emergency Use Authorization (EUA).
This is good and will increase the effective number of tests by at least a factor of 2-3 and perhaps more.
In other news, Representative Beyer (D-VA), Representative Gonzalez (R-OH) and Paul Romer have an op-ed calling for more prizes for testing:
Offering a federal prize solves a critical part of that problem: laboratories lack the incentive and the funds for research and development of a rapid diagnostic test that will, in the best-case scenario, be rendered virtually unnecessary in a year.
…We believe in the ability of the American scientific community and economy to respond to the challenge presented by the coronavirus. Congress just has to give them the incentive.
The National Institutes of Health (NIH) have already begun a similar strategy with their $1.4 billion “shark tank,” awarding speedy regulatory approval to five companies that can produce these tests. Expanding the concept to academic labs through a National Institute of Science and Technology (NIST)-sponsored competition has the added benefit ultimately funding more groundbreaking research once the prize money has been awarded.
This is all good but frustrating. I made the case for prizes in Grand Innovation Prizes for Pandemics in March and Tyler and I have been pushing for pooled testing since late March. We were by no means the first to promote these ideas. I am grateful things are happening and relative to normal procedure I know this is fast but in pandemic time it is molasses slow.
Vaccine Testing May Fail Without Human Challenge Trials
In Why Human Challenge Trials Will Be Necessary to Get a Coronavirus Vaccine I asked, “What if we develop a vaccine for COVID-19 but can’t find enough patients–healthy yet who might get sick–to run a randomized clinical trial?” Exactly that problem is now facing the Oxford vaccine in Britain.
An Oxford University vaccine trial has only a 50 per cent chance of success because coronavirus is fading so rapidly in Britain, a project co-leader has warned.
…Hill said that of 10,000 people recruited to test the vaccine in the coming weeks — some of whom will be given a placebo — he expected fewer than 50 people to catch the virus. If fewer than 20 test positive, then the results might be useless, he warned.
As I wrote, “A low infection rate is great, unless you want to properly test a vaccine.” Challenge trials have issues of external validity and they take time to setup properly but they produce results quickly and they can be especially useful in whittling down vaccine candidates to focus on the best candidates.
1DaySooner now has over 25 thousand volunteers from over 100 countries.
Rapid progress from Fast Grants
I was pleased to read this NYT reporting:
Yet another team has been trying to find drugs that work against coronavirus — and also to learn why they work.
The team, led by Nevan Krogan at the University of California, San Francisco, has focused on how the new coronavirus takes over our cells at the molecular level.
The researchers determined that the virus manipulates our cells by locking onto at least 332 of our own proteins. By manipulating those proteins, the virus gets our cells to make new viruses.
Dr. Krogan’s team found 69 drugs that target the same proteins in our cells the virus does. They published the list in a preprint last month, suggesting that some might prove effective against Covid-19…
It turned out that most of the 69 candidates did fail. But both in Paris and New York [where the drugs were shipped for testing], the researchers found that nine drugs drove the virus down.
“The things we’re finding are 10 to a hundred times more potent than remdesivir,” Dr. Krogan said. He and his colleagues published their findings Thursday in the journal Nature.
The Krogan team was an early recipient of Fast Grants, and you will find more detail about their work at the above NYT link. Fast Grants is also supporting Patrick Hsu and his team at UC Berkeley:
There are >100 antibody tests for coronavirus on the market. I explained https://t.co/fVV8ggQ5QI on @CNNTonight to @donlemon with @MarsonLab: what antibody tests can and cannot tell you, which tests perform well or don’t, and how scientists are racing to connect the dots https://t.co/uC5BMn726e
— Patrick Hsu (@pdhsu) April 30, 2020
And the work of the Addgene team:
Thanks #FastGrants (https://t.co/v7PtYNkOxG) for empowering us to continue supporting #COVID19 related reagent sharing. A thread, for some stats and shoutouts ⬇️ pic.twitter.com/eVKjbBguds
— Addgene (@Addgene) April 30, 2020
Early detection of superspreaders by mass group pool testing
Most of epidemiological models applied for COVID-19 do not consider heterogeneity in infectiousness and impact of superspreaders, despite the broad viral loading distributions amongst COVID-19 positive people (1-1 000 000 per mL). Also, mass group testing is not used regardless to existing shortage of tests. I propose new strategy for early detection of superspreaders with reasonable number of RT-PCR tests, which can dramatically mitigate development COVID-19 pandemic and even turn it endemic. Methods I used stochastic social-epidemiological SEIAR model, where S-suspected, E-exposed, I-infectious, A-admitted (confirmed COVID-19 positive, who are admitted to hospital or completely isolated), R-recovered. The model was applied to real COVID-19 dynamics in London, Moscow and New York City. Findings Viral loading data measured by RT-PCR were fitted by broad log-normal distribution, which governed high importance of superspreaders. The proposed full scale model of a metropolis shows that top 10% spreaders (100+ higher viral loading than median infector) transmit 45% of new cases. Rapid isolation of superspreaders leads to 4-8 fold mitigation of pandemic depending on applied quarantine strength and amount of currently infected people. High viral loading allows efficient group matrix pool testing of population focused on detection of the superspreaders requiring remarkably small amount of tests. Interpretation The model and new testing strategy may prevent thousand or millions COVID-19 deaths requiring just about 5000 daily RT-PCR test for big 12 million city such as Moscow.
Speculative, but I believe this is the future of our war against Covid-19.
The paper is by
