Results for “half doses” 45 found
Against Regulatory Nationalism
I’ve long argued that if a drug or medical device is approved in another country with a Stringent Regulatory Authority it ought to be approved in the United States. But, of course, the argument is even stronger in the other direction. Drugs and devices approved in the United States ought to be approved elsewhere. Indeed, this is how much of the world actually works because most countries do not have capability to evaluate drugs and devices the way the FDA or say the EMA does. Although it’s the way the world works, few will admit it because that would violate pretensions of regulatory nationalism. Moreover, keeping up with pretenses means transaction costs and unnecessary delays.
The price of such regulatory nationalism can be very high as indicated in this interview with Adar Poonawalla, chief executive of the Serum Institute of India (SII), the world’s largest producer of vaccines.
Some people think the reason that rollout has been slow in many countries is because the developers who hold the patents on the vaccines have licensed too few manufacturers to make them. Do you agree?
No. There are enough manufacturers, it just takes time to scale up. And by the way, I have been blown away by the cooperation between the public and private sectors in the last year, in developing these vaccines. What I find really disappointing, what has added a few months to vaccine delivery – not just ours – is the lack of global regulatory harmonisation. Over the last seven months, while I’ve been busy making vaccines, what have the US, UK and European regulators been doing? How hard would it have been to get together with the World Health Organization and agree that if a vaccine is approved in the half-dozen or so major manufacturing countries, it is approved to send anywhere on the planet?
Instead we have a patchwork of approvals and I have 70m doses that I can’t ship because they have been purchased but not approved. They have a shelf life of six months; these expire in April.
Did you get that? Regulatory nationalism has added months to vaccine delivery and now threatens to put to waste millions of stockpiled doses.
Addendum: See also Scott Sumner on the costs of regulatory nationalism.
Canada Needs a New Vaccination Strategy
The US vaccination rollout has been deadly slow, inefficient, and chaotic. It has also been one of the best in the world. Canada, for example, is far behind the US on vaccination.
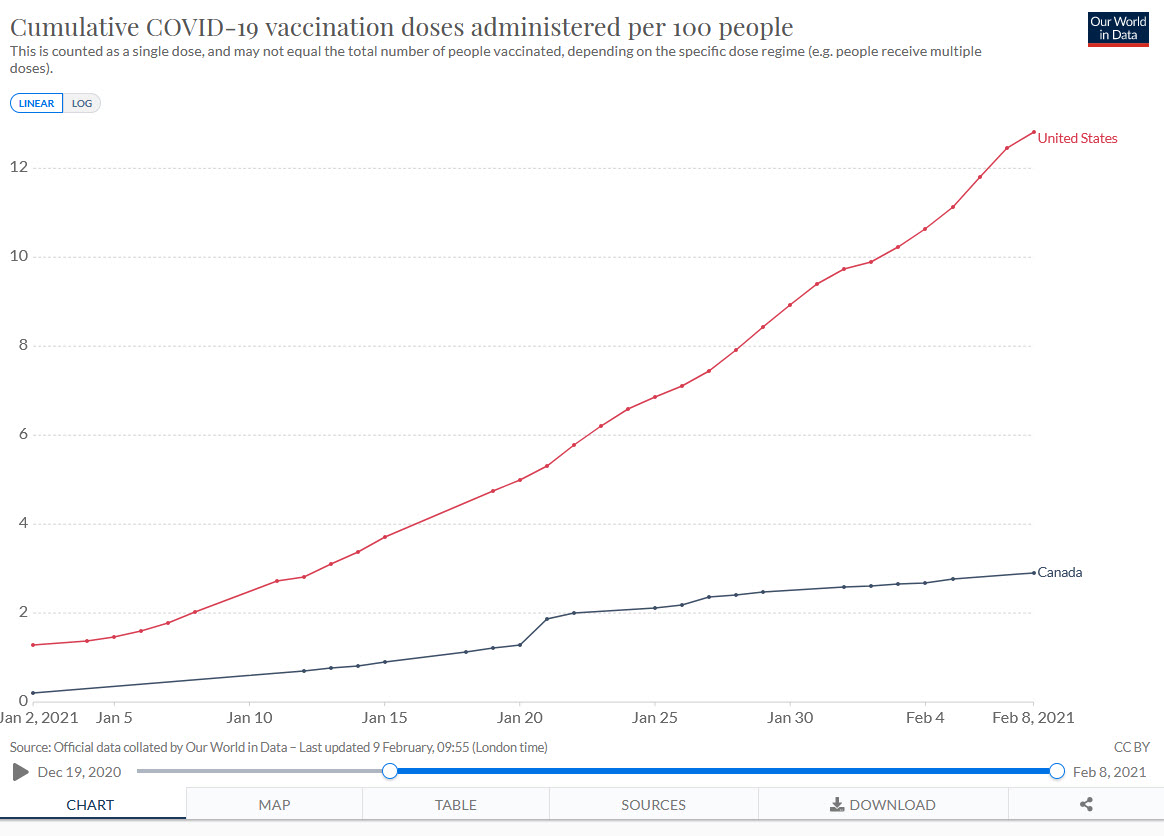
The Canadian deficit is mostly because they don’t have enough vaccine. Canada bought doses but they didn’t invest in capacity and a deal with China fell through. As a result, Canada won’t be getting lots of vaccine until March or April. Operation Warp Speed invested billions in the Modern vaccine and in early purchases of the Pfizer vaccine and thus got first dibs. The Americans are also not allowing vaccine to be exported to Canada. (We could at least give them access to our AstraZeneca factory!).
Regardless of blame, this puts Canada in a precarious situation. Death rates aren’t as high as in the United States but with new variants exploding, Canada is running a big risk. To get Canadians vaccinated more quickly–including my mother–Canada needs to find ways to stretch their vaccine supply–that means First Doses First, half dosing, intradermal delivery and other dose stretching strategies should be considered.
Many other countries are in a much more worse position than either the United States or Canada.
The Experts are Very Worried
Here is an interview with Dr. Peter Hotez, dean of the National School of Tropical Medicine at Baylor College of Medicine and the lead developer of a COVID vaccine being produced in India. He thinks the AstraZeneca vaccine should be approved immediately, as I have long argued.
President Biden himself announced Tuesday that we’re going to have maybe enough additional doses of the mRNA vaccines to fully vaccinate 300 million Americans by the end of summer or fall.
I’m saying, “Well, no, that’s that’s not gonna work.” Telling us “by the fall” is like telling us “when the glaciers are gonna come back down from Quebec.” I mean, that’s not adequate.
We’re going to have to figure out a way to vaccinate the American people by late spring. That’s a tall order. To beat back the virus we need to give two doses to three-quarters of the population, to 246 million Americans. That’s half a billion immunizations. To get there, we’d need a rate of immunizations two or three times higher than what’s proposed.
….We need vaccinations now.
..things have been slowed down with the AstraZeneca-Oxford adenovirus vaccine. My understanding is that the FDA insisted that they conduct a full-scale Phase Three trial in the U.S., and we won’t have results for that until April. Meanwhile, the European Medicines Agency, the EMA, is going to make a ruling on the AstraZeneca Oxford vaccine on Friday based on studies done in Europe and also probably on data from Brazil and South Africa. [The EMA authorized, AT].
Those are large, reliable studies?
Yeah….Because of these new variants, there’s great urgency here in the U.S. So I’m saying that sometimes we have to do things that take us out of our comfort zone in order to save lives. That means, rather than focusing only on the new study that we’re doing in the U.S., we also look at the dossier presented to the EMA.
As a regulatory agency the EMA is up there with our U.S. FDA. They’re the two best regulatory agencies in the world. So if they sign off, I think we should say, “Look, let’s do it. Let’s use that vaccine.”
We’ve already bought 300 million doses of the AstraZeneca-Oxford vaccine. We’ve paid for it — over a billion dollars — so let’s use it.
…And there’s also the recombinant protein vaccine our lab has developed at Baylor College of Medicine and Texas Children’s Hospital. In India they’re scaling that up to a billion doses. Nobody from the White House has approached us to say, “Hey, Peter, what can we do to bring that vaccine in.”
There seem to be blinders: All they can see is getting the mRNA vaccines. I don’t quite know what’s driving that. We have to figure out a way to bring the other ones on board.
And soon! We’re in the eye of the hurricane.
Hat tip: Jim Ward.
We Will Get to Herd Immunity in 2021…One Way or Another
By July it will all be over. The only question is how many people have to die between now and then?
Youyang Gu, whose projections have been among the most accurate, projects that the United States will have reached herd immunity by July, with about half of the immunity coming from vaccinations and half from infections. Long before we reach herd immunity, however, the infection and death rates will fall. Gu is projecting that by March infections will be half what they are now and by May about one-tenth the current rate. The drop will catch people by surprise just like the increase. We are not good at exponentials. The economy will boom in Q2 as infections decline.
If that sounds good bear in mind that 400,000 people are dead already and the CDC expects another 100,000 dead by February. We have a very limited window in the United States to make a big push on vaccines and we are failing. We are failing phenomenally badly.
To understand how bad we are failing compare with flu vaccinations. Every year the US gives out about 150 million flu vaccinations within the space of about 3 months or 1.6 million shots a day. Thus, we vaccinate for flu at more than twice the speed we are vaccinating for COVID! Yes, COVID vaccination has its own difficulties but this is an emergency with tens of thousands of lives at stake.
I would love it if we mobilized serious resources and vaccinated at Israel’s rate–30% of the population in a month. But if we simply vaccinated for COVID at the same rate as we do for flu we would save thousands of lives and hundreds of billions of dollars in GDP. The comparison with flu vaccinations also reminds us that we don’t necessarily need the National Guard or mass clinics in stadiums. Use the HMOs and the pharmacies!
And let’s make it easier for the pharmacies. It’s beyond ridiculous that we are allowing counties to set their own guidelines for who should be vaccinated first. We need one, or at most 50, set of guidelines and lets not worry so much at people jumping the queue. (The ones jumping the queue are probably the ones who want to get back to the bars and social life the most so vaccinating them first has some side benefits.)
Of course, the faster we vaccinate the more vaccine quantities will become the binding constraint which is why we also need to approve more vaccines, move to First Doses First (delay second doses like the British), and use Moderna half-doses. Fire on all cylinders!
Time is of the essence.
Hat tip: Kevin Bryan and Witold Wiecek.
Approve the AstraZeneca Vaccine Now!
Here’s Marty Makary, M.D., a professor of surgery and health policy at the Johns Hopkins University School of Medicine:
Finally, the FDA needs to stop playing games and authorize the Oxford-AstraZeneca vaccine. It’s safe, cheap ($2-$3 a dose), and is the easiest vaccine to distribute. It does not require freezing and is already approved and being administered in the United Kingdom.
Sadly, the FDA is months away from authorizing this vaccine because FDA career staff members insisted on another clinical trial to be completed and are punishing the company for inadvertently giving a half-dose of the vaccine to some people in the trial.
It’s like the FDA is holding out, pontificating existing excellent data and being vindictive against a company for making a mistake while thousands of Americans die each day.
Ironically, those in the Oxford-AstraZeneca trial who inadvertently received half the initial vaccine dose had lower infection rates. And this week Dr. Moncef Slaoui, the chief adviser to Operation Warp Speed, acknowledged that using half a dose might be a good broader strategy for the U.S. to double our supply as long our supply is severely constrained. That’s a good strategy that makes sense.
See also my post The AstraZeneca Factory in Baltimore. Thousands of people are dying every day. We have a vaccine factory ready to go. The FDA should lifts its ban on the AstraZeneca vaccine.
Be Prepared! Sars-COV-3
The federal government was unprepared for the pandemic, despite multiple, loud and clear warnings. State and local governments were unprepared for vaccines, despite multiple, loud and clear warnings. The Capitol Police were unprepared for rioters, despite multiple, loud and clear warnings.
The record isn’t good but as a Queen’s Scout I persist. We now have multiple, loud and clear warnings that new variants of the SARS-COV II virus are more transmissible and thus much more dangerous. But we can do something. As wrote in The New Strain and the Need for Speed
One of the big virtues of mRNA vaccines is that much like switching a bottling plant from Sprite to 7-Up we could tweak the formula and produce a new vaccine using exactly the same manufacturing plants. Moreover, Marks and Hahn at the FDA have said that the FDA would not require new clinical trials for safety and efficacy just smaller, shorter trials for immune response (similarly we don’t do new large-scale clinical trials for every iteration of the flu vaccine.) Thus, if we needed it, we could modify mRNA vaccines (not other types) for a new variant in say 8-12 weeks.
Thus, let’s start doing much more sequencing to discover new strains–and also think about potential new strains–and start phase I and phase II trials of new vaccines. Florian Krammer suggested an even more ambitious plan to do the same thing for all potential pandemic viruses:
From each of the identified virus families, which should certainly include the Paramyxoviridae, Orthomyxoviridae, and Coronaviridae families, a handful of representative strains with the highest pandemic potential should be selected for vaccine production. Up to 50–100 different viruses could be selected and this would broadly cover all phylogenies that may give rise to pandemic strains….It should be possible to choose candidates that are close to viruses that might emerge in the human population. The idea is that once viruses are selected, vaccines can be produced in different platforms and tested in phase 1 and phase 2 trials with some of the produced vaccine being stockpiled. This would likely cost 20–30 million US dollars per vaccine candidate resulting in a cost of 1–3 billion US dollars.
What I am suggesting is less ambitious–just do this for Sars-COV-3, 4, 5 and 6. But do it now!
Hat tip: Daniel Bier.
Broken Record Addendum: We should make better use of our limited vaccine supply by moving to First Doses First and/or fractional dosing and approve the AstraZeneca vaccine immediately and spend billions to increase the rate of vaccinations and to speed new vaccines (such as those from J&J and Novavax) to market.
“New York’s mass-vaccination plans are shelved as Cuomo takes different path”
County officials who have for years been planning for a mass vaccination said they are seeing that training and preparation — much of it funded by millions of dollars in federal grants — pushed aside as the administration of Gov. Andrew M. Cuomo has retained control of the state’s coronavirus vaccination program, including having hospitals rather than local health departments administer the doses.
Interviews with multiple county officials over the past week confirm that many are unclear why the governor’s administration has not activated the county-by-county system, a plan that included recent practice sessions in which members of the public received regular flu vaccines at drive-thru sites.
…In Albany County, officials have privately said they could vaccinate the population of the southern half of the county in a few days if they were given the coronavirus vaccines and allowed to mobilize their plan.
Here is the full, gory story. It is clear they have just begun thinking about this. I really do not understand why Paul Krugman has been praising the New York State response for so many months, they have gone from one disgrace to another. Ross Barkan offers further commentary. And here is de Blasio on Cuomo.
As a side note this is interesting: “The Times Union is not disclosing the [vaccination] location because county officials contend the vaccination sites should not be publicly disclosed for security purposes.”
To be clear, other states are messing up too, some of them worse than NY.
The New Strain and the Need for Speed
I was going to write a long blog post on the new strain but Zeynep Tufekci has written an excellent piece for The Atlantic. I will quote from it and add a few points.
One of the big virtues of mRNA vaccines is that much like switching a bottling plant from Sprite to 7-Up we could tweak the formula and produce a new vaccine using exactly the same manufacturing plants. Moreover, Marks and Hahn at the FDA have said that the FDA would not require new clinical trials for safety and efficacy just smaller, shorter trials for immune response (similarly we don’t do new large-scale clinical trials for every iteration of the flu vaccine.) Thus, if we needed it, we could modify mRNA vaccines (not other types) for a new variant in say 8-12 weeks. As Zeynep notes, however, the vaccines are very likely to work well for the new variant. It’s nice to know, however, that we do have some flexibility.
The real worry is not that the vaccines won’t work but that we won’t get them into arms fast enough. We were already going too slow but in a race against the new more transmissible variant we are looking like tortoises.
A more transmissible variant of COVID-19 is a potential catastrophe in and of itself. If anything, given the stage in the pandemic we are at, a more transmissible variant is in some ways much more dangerous than a more severe variant. That’s because higher transmissibility subjects us to a more contagious virus spreading with exponential growth, whereas the risk from increased severity would have increased in a linear manner, affecting only those infected.
Here’s a key example from epidemiologist Adam Kucharski:
As an example, suppose current R=1.1, infection fatality risk is 0.8%, generation time is 6 days, and 10k people infected (plausible for many European cities recently). So we’d expect 10000 x 1.1^5 x 0.8% = 129 eventual new fatalities after a month of spread. What happens if fatality risk increases by 50%? By above, we’d expect 10000 x 1.1^5 x (0.8% x 1.5) = 193 new fatalities.
Now suppose transmissibility increases by 50%. By above, we’d expect 10000 x (1.1 x 1.5)^5 x 0.8% = 978 eventual new fatalities after a month of spread.
…the key message: an increase in something that grows exponentially (i.e. transmission) can have far more effect than the same proportional increase in something that just scales an outcome (i.e. severity).
I argued that the FDA should have approved the Pfizer vaccine, on a revocable basis, as soon as the data on the safety and efficacy of its vaccine were made available around Nov. 20. But the FDA scheduled it’s meeting of experts for weeks later and didn’t approve until Dec. 11, even as thousands of people were dying daily. We could have been weeks ahead of where we are today. Now the epidemiologists are telling us that weeks are critical. As Zeynep notes holding back second doses looks like a clear mistake and the balance of the evidence also suggests we should move to first doses first:
All this means that the speed of the vaccine rollout is of enormous importance.
…Meanwhile, the United States was reportedly planning to hold back half the vaccine it has in freezers as a hedge against supply-chain issues, and some states may be slowed down by murky prioritization plans. Scott Gottlieb—the former FDA chief and a current board member of Pfizer—has argued that the U.S. should also go ahead with vaccinating as many people as possible right now and trust that the supply chain will be there for the booster. Researchers in Canada—where some provinces decided to vaccinate now as much as possible without holding half in reserve, and will administer the booster with future supplies—estimate that this type of front-loading can help “avert between 34 and 42 per cent more symptomatic coronavirus infections, compared with a strategy of keeping half the shipments in reserve.” (Note that this strategy, which is different from the one the United Kingdom just announced it will adopt in prioritizing the first dose, does not even necessarily involve explicitly changing booster timing protocols in order to maximize vaccination now; it just means not waiting to get shots into arms when the vaccines are currently available.) These were already important conversations to have, but given the threat posed by this new variant, they are even more urgent.
Perhaps most critically, the FDA should approve the AstraZeneca vaccine if not as part of Operation Warp Speed then on a right to try basis. We need every weapon in the arsenal. How many times must we learn not to play with exponential matches?
Addendum: See also this excellent Miles Kimball post, How Perfectionism Has Made the Pandemic Worse.
Wise Canadians
On December 12 I wrote:
We should vaccinate 6 million people with first dose NOW. It is deadly cautious to hold second dose in *reserve*. Supply chain will be ok and the exact timing of the second dose is not magical and likely not critical.
Modelling by a group at the University of Toronto confirmed.
Ashleigh Tuite, an epidemiologist at U of T’s Dalla Lana School of Public Health….said she and her colleagues projected that frontloading vaccine doses would avert between 34 and 42 per cent more symptomatic coronavirus infections, compared with a strategy of keeping half the shipments in reserve.
“It makes much more sense to just get as many people their first doses as soon as possible,” Dr. Tuite said.
…everyone should get the second dose on schedule, but if supply issues delay that injection by a week or two, it shouldn’t hamper how well the vaccines work.
According to Abigail Bimman at Global News, Ontario will now switch to getting as many first doses out as possible:
NEW: Ontario is changing its vaccine policy and no longer reserving second doses, but getting all of the initial 90k out the door- they expect to finish them in the “next several days” – Health Minister’s office tell @globalnew. Change due to confidence in supply chain.
It’s not all the way to first doses first but it’s a minimally risky, smart move. Indeed, Nova Scotia, Saskatchewan and British Columbia already have said that they won’t hold back first doses.
The United States should listen to the wise Canadians.
Double the Inoculated Population with One Dose
I’ve been arguing that we should delay the second dose (or at least not hold back first doses) in order to hit the virus hard and inoculate more people on the first dose. I wrote:
We should vaccinate 6 million people with first dose NOW. It is deadly cautious to hold second dose in *reserve*. Supply chain will be ok and the exact timing of the second dose is not magical and likely not critical. In the accidental low-dose, standard-dose regime for the AZ vaccine, people got the second dose 7 to 8 weeks after the first dose and that was the 90% efficacious regime. [A different vaccine obviously but ] Exact timing of the second-dose does not seem critical, although everyone should get a second dose.
Today epidemiologist Michael Mina and writer Zeynep Tufekci, who has been ahead of the curve on much of the discussion, make the case even more strongly in the NYTimes:
First, the science. While the vaccine trials were designed to evaluate a two-dose regimen, some immunity might be acquired before a second dose is administered. We know, for instance, that a Covid-19 infection appears to yield protection for at least six months. While infections are not vaccinations, and while we need more data on this, it’s plausible that the immunity gained from a vaccination may turn out to be even stronger than what comes from an infection. The reason we do a second — booster — vaccination is that these later doses help to solidify immune memory, in part by giving extra training to the cells that produce antibodies, a process called affinity maturation. But this process begins with the single dose, and the evidence collected between the time of the first and second doses in tens of thousands of people in the Phase 3 trials suggests that the level of affinity maturation may provide enough protection to meet the standards we have set for vaccine approval during this pandemic even without the second dose.
While we know that the single dose can protect against disease, we don’t yet know how long this immune protection will last, and at what level. However, there is no rule that says that vaccines must be boosted within weeks of each other. For measles, the booster dose is given years after the first dose. If the booster dose could be given six months or a year after the first dose, while maintaining high efficacy before the second dose, that would allow twice as many people to get vaccinated between now and later next year, accelerating herd immunity — greatly helping end the crisis phase of the pandemic in the United States.
… we should begin immediate single-dose trials, recruiting volunteers from low-risk populations who are first in line for the vaccinations. For example, among health care workers protective equipment works, rates of infection among this group have fallen sharply and severe disease is much more rare.Younger essential workers without risk factors are less likely to be severely affected if they are exposed since this disease’s impact rises steeply with age. Just as tens of thousands of people volunteered for the earlier vaccine trials, many may well volunteer to test a placebo against a second dose, allowing us to quickly ascertain questions of durability and effectiveness of the single dose.
Two additional points. First, mix and match, as I argued earlier, may be beneficial:
…we could mix and match vaccines. The UK will run a trial on this question. Mix and matching has two potentially good properties. First, mix and matching could make the immune system response stronger than either vaccine alone because different vaccines stimulate the immune system in different ways. Second, it could help with distribution. It’s going to be easier to scale up the AZ vaccine than the mRNA vaccines, so if we can use both widely we can get more bang for our shot.
Second, an economics issue. If we want Pfizer and Moderna on board we need to pay them not just to run the clinical trials but to be happy with potentially selling half as many doses. Incentives matter.
The Simple Math of FDA Delay
Two to three thousand people a day are dying from COVID. Thus anything that delays rolling out a vaccine has a very high cost in human lives. People want to deny this, perhaps because it is so horrifying. I get a lot of pushback when I say that FDA delay is deadly. Let’s dispense with a few objections. It is true, of course, that the people who are dying today can’t literally be saved by a vaccine today but they could have been saved had they been vaccinated four or five weeks ago and similarly projecting forward.
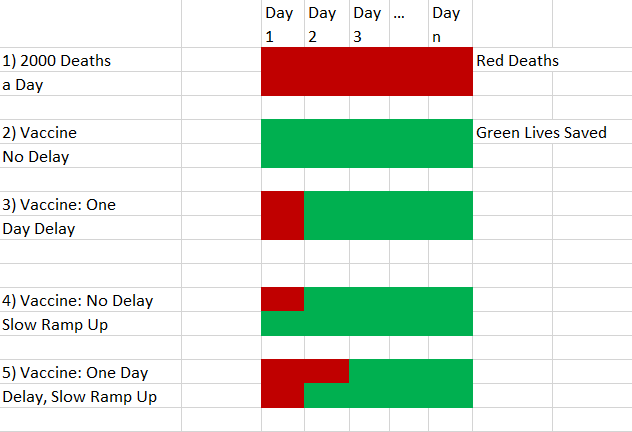
Another response that many smart people tell me is that a vaccine can’t be rolled out immediately so even under the best scenarios you couldn’t save that many people immediately. That’s true but irrelevant. Since a lot of people are getting this wrong, I want to show this in a simple model using pictures. Red is for deaths. Green is for life. Suppose two thousand people are dying from COVID a day as in panel 1. Let’s for the sake of the simple model assume that you could deliver a vaccine to everyone on Day 1. You would then save 2000 lives a day going forward for however long the pandemic would have lasted as shown in panel 2. If you delay by one day then two thousand people die who would have lived without the delay, as shown in panel 3. Pretty obvious so far.
Now assume that the vaccine can’t roll out to everyone immediately. For the sake of this simple model let’s assume that on day one you can only vaccinate half the population. By doing so you save 1000 lives on day 1 and 2000 lives every day thereafter for the length of the pandemic. That’s the fourth panel. Now suppose we delay the vaccine rollout by one day. 2000 people die on Day 1 but you save 1000 on Day 2 and 2000 on Day 3 and every day thereafter for the length of the pandemic. How many people were killed by the delay? Compare the 4th and 5th panels. 2000 exactly as before! The slow ramp up doesn’t change the number of deaths caused by delay it just spreads them out over different days. You can adjust the ramp so that it occurs over 10 days or 30 days. Doesn’t change much on the delay margin unless you delay for so long that the pandemic is close to being over.
What could matter is if delay increases the speed at which you can ramp up. I doubt that this is true. We were ready to go with millions of doses in late October (guess why?). (In fact we had a vaccine in January and millions of doses around March-April.) We won’t really be better prepared tomorrow than we are today. It’s learning by doing that matters. See the point Tyler made earlier about economic time versus calendar time.
As Tyler noted, this is hardly the final analysis but many people are not even conceptualizing the problem correctly and this is a good place to begin.
My spring 2020 Industrial Organization reading list and syllabus
It is long, do note that many topics are covered in the other half of the class, I tried to put this beneath the fold, but today WordPress software is not cooperating…
- Productivity
American Economic Review Symposium, May 2010, starts with “Why do Firms in Developing Countries Have Low Productivity?” runs pp.620-633.
Nicholas Bloom, Raffaella Sadun, and John Van Reenen, “Recent Advances in the Empirics of Organizational Economics,” http://cep.lse.ac.uk/pubs/download/dp0970.pdf.
Serguey Braguinsky, Lee G. Branstetter, and Andre Regateiro, “The Incredible Shrinking Portuguese Firm,” http://papers.nber.org/papers/w17265#fromrss.
Bloom, Nicholas, Raffaella Sadun, and John Van Reenen. “Management as a Technology?” National Bureau of Economic Research working paper 22327, June 2016.
David Lagakos, “Explaining Cross-Country Productivity Differences in Retail Trade,” Journal of Political Economy, April 2016, 124, 2, 1-49.
Dani Rodrik, “A Surprising Convergence Result,” http://rodrik.typepad.com/dani_rodriks_weblog/2011/06/a-surprising-convergence-result.html, and his paper here http://www.hks.harvard.edu/fs/drodrik/Research%20papers/The%20Future%20of%20Economic%20Convergence%20rev2.pdf
Tyler Cowen, The Complacent Class, chapter four, “Why Americans Stopped Creating,” 2017.
Ufuk Akcigit and Sina T. Ates, “Ten Facts on Declining Business Dynamism and Lessons from Endogenous Growth Theory,” NBER working paper 25755, April 2019.
Syerson, Chad “What Determines Productivity?” Journal of Economic Literature, June 2011, XLIX, 2, 326-365.
Michael Kremer, “The O-Ring Theory of Economic Development,” Quarterly Journal of Economics, August 1993, 108, 3, 551-575.
Song, Jae, David J. Price, Fatih Guvenen, and Nicholas Bloom. “Firming Up Inequality,” Federal Reserve Bank of Minneapolis working paper 750, April 2018. Do not bother with the very long appendix.
Nicholas Bloom, Raffaella Sadun, and John Van Reenen, the slides for “Americans do I.T. Better: US Multinationals and the Productivity Miracle,” http://www.people.hbs.edu/rsadun/ADITB/ADIBslides.pdf, the paper is here http://www.stanford.edu/~nbloom/ADIB.pdf but I recommend focusing on the slides.
Tyler Cowen and Ben Southwood, “Is the rate of scientific progress slowing down?”, https://docs.google.com/document/d/1cEBsj18Y4NnVx5Qdu43cKEHMaVBODTTyfHBa8GIRSec/edit
Patrick Collison and Michael Nielsen, “Science is Getting Less Bang for its Buck,” Atlantic, November 16, 2018, https://www.theatlantic.com/science/archive/2018/11/diminishing-returns-science/575665/
Decker, Ryan and John Haltiwanger, Ron S. Jarmin, and Javier Miranda. “Where Has all the Skewness Gone? The Decline in High-Growth (Young) Firms in the U.S. National Bureau of Economic Research working paper 21776, December 2015.
Furman, Jason and Peter Orszag. “A Firm-Level Perspective on the Role of Rents in the Rise in Inequality.” October 16, 2015.
2. Competition and monopoly
Bresnahan, Timothy F. “Competition and Collusion in the American Automobile Industry: the 1955 Price War,” Journal of Industrial Economics, 1987, 35(4), 457-82.
Asker, John, “A Study of the Internal Organization of a Bidding Cartel,” American Economic Review, (June 2010), 724-762.
Tim Sablik and Nicholas Trachter, “Are Markets Becoming Less Competitive?” Economic Brief, Federal Reserve Bank of Richmond, June 2019.
Susanto Basu, “Are Price-Cost Markups Rising in the United States? A Discussion of the Evidence,” Journal of Economic Perspectives, Summer 2019, 33, 3, 3-22.
Esteban Rossi-Hansberg, Pierre-Daniel Sarte, and Nicholas Trachter, “Diverging Trends in National and Local Concentration,” NBER Working Paper 25066, Septemmber 2018.
David Autor, David Dorn, Lawrence Katz, Christina Patterson, John Van Reenen, “The Fall of the Labor Share and the Rise of Superstar Firms,” https://economics.mit.edu/files/12979, make sure you get the Oct. 2019 version, not the earlier NBER paper.
Whinston, Michael D., “Antitrust Policy Toward Horizontal Mergers,” Handbook of Industrial Organization, vol.III, chapter 36, see also chapter 35 by John Sutton.
Jan De Loecker and Jan Eeckhout, “The Rise of Market Power and its Macroeconomic Implications,” http://www.janeeckhout.com/wp-content/uploads/RMP.pdf. My comment on it is here: https://marginalrevolution.com/marginalrevolution/2017/08/rise-market-power.html and see also me on intangible capital, https://marginalrevolution.com/marginalrevolution/2017/09/intangible-investment-monopoly-profits.html.
Chang-Tai Hsieh and Esteban Rossi-Hansberg, “The Industrial Revolution in Services, September 20, 2019, on-line.
Klein, Benjamin and Leffler, Keith. “The Role of Market Forces in Assuring Contractual Performance.” Journal of Political Economy 89 (1981): 615-641.
Breit, William. “Resale Price Maintenance: What do Economists Know and When Did They Know It?” Journal of Institutional and Theoretical Economics (1991).
Bogdan Genchev, and Julie Holland Mortimer. “Empirical Evidence on Conditional Pricing Practices.” NBER working paper 22313, June 2016.
Sproul, Michael. “Antitrust and Prices.” Journal of Political Economy (August 1993): 741-754.
McCutcheon, Barbara. “Do Meetings in Smoke-Filled Rooms Facilitate Collusion?” Journal of Political Economy (April 1997): 336-350.
Crandall, Robert and Winston, Clifford, “Does Antitrust Improve Consumer Welfare?: Assessing the Evidence,” Journal of Economic Perspectives (Fall 2003), 3-26, available at http://www.brookings.org/views/articles/2003crandallwinston.htm.
FTC, Bureau of Competition, website, http://www.ftc.gov/bc/index.shtml, an optional browse, perhaps read about some current cases and also read the merger guidelines.
Parente, Stephen L. and Prescott, Edward. “Monopoly Rights: A Barrier to Riches.” American Economic Review 89, 5 (December 1999): 1216-1233.
Demsetz, Harold. “Why Regulate Utilities?” Journal of Law and Economics (April 1968): 347-359.
Armstrong, Mark and Sappington, David, “Recent Developments in the Theory of Regulation,” Handbook of Industrial Organization, chapter 27, also on-line.
Shleifer, Andrei. “State vs. Private Ownership.” Journal of Economic Perspectives (Fall 1998): 133-151.
Xavier Gabaix and David Laibson, “Shrouded Attributes, Consumer Myopia, and Information Suppression in Competitive Markets,”http://papers.ssrn.com/sol3/papers.cfm?abstract_id=728545.
Strictly optional, most of you shouldn’t read this: Ariel Pakes and dynamic computational approaches to modeling oligopoly:
http://www.economics.harvard.edu/faculty/pakes/files/Pakes-Fershtman-8-2010.pdf
http://www.economics.harvard.edu/faculty/pakes/files/handbookIO9.pdf
III. Economics of Tech
Farrell, Joseph and Klemperer, Paul, “Coordination and Lock-In: Competition with Switching Costs and Network Effects,” Handbook of Industrial Organization, vol.III, chapter 31, also on-line.
Weyl, E. Glenn. “A Price Theory of Multi-Sided Platforms.” American Economic Review, September 2010, 100, 4, 1642-1672.
Gompers, Paul and Lerner, Josh. “The Venture Capital Revolution.” Journal of Economic Perspectives (Spring 2001): 145-168.
Paul Graham, essays, http://www.paulgraham.com/articles.html, to browse as you find useful or not.
Acemoglu, Daron and Autor, David, “Skills, Tasks, and Technologies: Implications for Employment and Earnings,” http://econ-www.mit.edu/files/5607
Robert J. Gordon and Ian Dew-Becker, “Unresolved Issues in the Rise of American Inequality,” http://www.people.fas.harvard.edu/~idew/papers/BPEA_final_ineq.pdf
Browse through the first issue of Nakamoto.com on blockchain governance, read (or not) as you find useful.
IV. Organization and capital structure
Ronald Coase and Oliver Williamson on the firm, if you haven’t already read them, but limited doses should suffice.
Gibbons, Robert, “Four Formal(izable) Theories of the Firm,” on-line at http://papers.ssrn.com/sol3/papers.cfm?abstract_id=596864.
Van den Steen, Eric, “Interpersonal Authority in a Theory of the Firm,” American Economic Review, 2010, 100:1, 466-490.
Lazear, Edward P. “Leadership: A Personnel Economics Approach,” NBER Working Paper 15918, 2010.
Oyer, Paul and Schaefer, Scott, “Personnel Economics: Hiring and Incentives,” NBER Working Paper 15977, 2010.
Tyler Cowen chapter on CEO pay in big Business, to be distributed.
Ben-David, Itzhak, and John R. Graham and Campbell R. Harvey, “Managerial Miscalibration,” NBER working paper 16215, July 2010.
Glenn Ellison, “Bounded rationality in Industrial Organization,” http://cemmap.ifs.org.uk/papers/vol2_chap5.pdf
Miller, Merton, and commentators. “The Modigliani-Miller Propositions After Thirty Years,” and comments, Journal of Economic Perspectives (Fall 1988): 99-158.
Myers, Stewart. “Capital Structure.” Journal of Economic Perspectives (Spring 2001): 81-102.
Hansemann, Henry. “The Role of Non-Profit Enterprise.” Yale Law Journal (1980): 835-901.
Kotchen, Matthew J. and Moon, Jon Jungbien, “Corporate Social Responsibility for Irresponsibility,” NBER working paper 17254, July 2011.
Strictly optional but recommended for the serious: Ponder reading some books on competitive strategy, for MBA students. Here is one list of recommendations: http://www.linkedin.com/answers/product-management/positioning/PRM_PST/20259-135826
Furman, Jason. ”Business Investment in the United States: Facts, Explanations, Puzzles, and Policy.” Remarks delivered at the Progressive Policy Institute, September 30, 2015, on-line at https://m.whitehouse.gov/sites/default/files/page/files/20150930_business_investment_in_the_united_states.pdf.
Scharfstein, David S. and Stein, Jeremy C. “Herd Behavior and Investment.” American Economic Review 80 (June 1990): 465-479.
Stein, Jeremy C. “Efficient Capital Markets, Inefficient Firms: A Model of Myopic Corporate Behavior.” Quarterly Journal of Economics 104 (November 1989): 655-670.
V. Sectors: finance, health care, education, international trade, others
Gorton, Gary B. “Slapped in the Face by the Invisible Hand: Banking and the Panic of 2007,” http://papers.ssrn.com/sol3/papers.cfm?abstract_id=1401882, published on-line in 2009.
Erel, Isil, Nadault, Taylor D., and Stulz, Rene M., “Why Did U.S. Banks Invest in Highly-Rated Securitization Tranches?” NBER Working Paper 17269, August 2011.
Healy, Kieran. “The Persistence of the Old Regime.” Crooked Timber blog, August 6, 2014.
More to be added here, depending on your interests.
Chernobyl
 Chernobyl, HBO’s taut 5-part mini-series, is excellent and it sticks close to the facts (although one female character played by Emily Watson is clearly made up). By all accounts, the series accurately represents life in the former Soviet Union and through a variety of means from color palette to casting and dialogue it does a remarkable job at capturing the political economy. One thing I learned (so far, it hasn’t all appeared yet) is that it could have been much, much worse but the Russians avoided the worst scenario with a combination of bravery, smarts and luck.
Chernobyl, HBO’s taut 5-part mini-series, is excellent and it sticks close to the facts (although one female character played by Emily Watson is clearly made up). By all accounts, the series accurately represents life in the former Soviet Union and through a variety of means from color palette to casting and dialogue it does a remarkable job at capturing the political economy. One thing I learned (so far, it hasn’t all appeared yet) is that it could have been much, much worse but the Russians avoided the worst scenario with a combination of bravery, smarts and luck.
The number of cancer deaths from Chernobyl appears to be quite low. The WHO estimated an additional 9,335 deaths with about half of those coming from workers and nearby residents and other half more distant impacts, other estimates are higher. More recent analysis, however, suggests that Chernobyl and its aftermath had relatively small but significant effects across a large number of people. Here are two recent papers:
Chernobyl’s Subclinical Legacy: Prenatal Exposure to Radioactive Fallout and School Outcomes in Sweden by Almond, Edlund and Palme.
Abstract: We use prenatal exposure to Chernobyl fallout in Sweden as a natural experiment inducing variation in cognitive ability. Students born in regions of Sweden with higher fallout performed worse in secondary school, in mathematics in particular. Damage is accentuated within families (i.e., siblings comparison) and among children born to parents with low education. In contrast, we detect no corresponding damage to health outcomes. To the extent that parents responded to the cognitive endowment, we infer that parental investments reinforced the initial Chernobyl damage. From a public health perspective, our findings suggest that cognitive ability is compromised at radiation doses currently considered harmless.
and The long-run consequences of Chernobyl: Evidence on subjective well-being, mental health and welfare by Danzer and Danzer.
Abstract: This paper assesses the long-run toll taken by a large-scale technological disaster on welfare, well-being and mental health. We estimate the causal effect of the 1986 Chernobyl catastrophe after 20 years by linking geographic variation in radioactive fallout to respondents of a nationally representative survey in Ukraine according to their place of residence in 1986. We exclude individuals who were exposed to high levels of radiation—about 4% of the population. Instead, we focus on the remaining majority of Ukrainians who received subclinical radiation doses; we find large and persistent psychological effects of this nuclear disaster. Affected individuals exhibit poorer subjective well-being, higher depression rates and lower subjective survival probabilities; they rely more on governmental transfers as source of subsistence. We estimate the aggregate annual welfare loss at 2–6% of Ukraine’s GDP highlighting previously ignored externalities of large-scale catastrophes.
Hat tip: Jennifer Doleac and Wojtek Kopczuk.
Are the people in Middletown, Ohio “killers”?
As their budgets strain, communities have begun questioning how much money and effort they should be spending to deal with overdoses, especially in cases involving people who have taken near-fatal overdoses multiple times. State and local officials say it might be time for “tough love”: pushing soaring medical costs onto drug abusers or even limiting how many times first responders can save an individual’s life.
“It’s not that I don’t want to treat overdose victims, it’s that the city cannot afford to treat overdose victims,” said Middletown Council Member Daniel Picard, noting this industrial town in northern Butler County might have to raise taxes in response to the crisis.
Often, the only thing separating whether an overdose victim goes to the hospital instead of the morgue is a dose of naloxone, also known by the brand name Narcan, a medication that can reverse the effects of opioid overdoses.
Two doses of an injectable form of naloxone, Evzio, cost $4,500, up from $690 in 2014. The price of other forms of the drug, including the nasally administered Narcan, typically range from $70 to $150 per dose, officials say.
…Here in Ohio, first responders say it’s not uncommon for overdose victims to have previously been revived with naloxone at least a half-dozen times.
…Picard, the council member, has proposed a controversial three-strikes policy in which first responders wouldn’t administer Narcan to repeated overdose victims.
Here is the Tim Craig at WaPo story. I do not know what is the proper response to such opioid cases, or how much money should be spent. I do know that somewhere, somehow a line has to be drawn. And if you are reading a discussion of health care policy that does not acknowledge such a line, and set out possible standards for it, beware of sophistry and illusion.
More Lead, More Crime
In the second half of the nineteenth century, many American cities built water systems using lead or iron service pipes. Municipal water systems generated significant public health improvements, but these improvements may have been partially offset by the damaging effects of lead exposure through lead water pipes. We study the effect of cities’ use of lead pipes on homicide between 1921 and 1936. Lead water pipes exposed entire city populations to much higher doses of lead than have previously been studied in relation to crime. Our estimates suggest that cities’ use of lead service pipes considerably increased city-level homicide rates.
That’s from Feigenbaum and Muller in Lead Exposure and Violent Crime in the Early Twentieth Century. Lead, it ain’t just about Flint.