Results for “Tests” 772 found
Do protests matter?
Only rarely:
Recent social movements stand out by their spontaneous nature and lack of stable leadership, raising doubts on their ability to generate political change. This article provides systematic evidence on the effects of protests on public opinion and political attitudes. Drawing on a database covering the quasi-universe of protests held in the United States, we identify 14 social movements that took place from 2017 to 2022, covering topics related to environmental protection, gender equality, gun control, immigration, national and international politics, and racial issues. We use Twitter data, Google search volumes, and high-frequency surveys to track the evolution of online interest, policy views, and vote intentions before and after the outset of each movement. Combining national-level event studies with difference-in-differences designs exploiting variation in local protest intensity, we find that protests generate substantial internet activity but have limited effects on political attitudes. Except for the Black Lives Matter protests following the death of George Floyd, which shifted views on racial discrimination and increased votes for the Democrats, we estimate precise null effects of protests on public opinion and electoral behavior.
That is from a new NBER working paper by Amory Gethin and Vincent Pons.
Don’t Let the FDA Regulate Lab Tests!
I have been warning about the FDA’s power grab over lab developed tests. Lab developed tests have never been FDA regulated except briefly during the pandemic emergency when such regulation led to catastrophic consequences. Catastrophic consequences that had been predicted in advanced by Paul Clement and Lawrence Tribe. Despite this, for reasons I do not understand, the FDA plan is marching forward but many other people are starting to warn of dire consequences. Here, for example, is the executive summary from a letter by ARUP Laboratories, a non-profit enterprise of the University of Utah Department of Pathology:
ARUP urges the FDA to withdraw the proposed rule:
- The FDA proposal will reduce, an in many cases eliminate, access to safe and essential testing services, particularly for patients with rare diseases.
- Laboratory-developed tests are not devices as defined by the Medical Device Amendments of 1976, nor are clinical laboratories acting as manufacturers.
- The FDA does not have the statutory authority to regulate laboratory-developed tests.
- The FDA does not have the authority to regulate states, or state-owned entities. This is particularly relevant for the proposed rule regarding academic medical centers.
- The FDA’s regulatory impact analysis is flawed in its design, source information, methods. and conclusions, and it systematically overestimates purported benefits of the proposed rule and dramatically underestimates its cost to society, the healthcare industry, and the ability to provide ongoing essential laboratory services to patients.
- The proposed rule would significantly limit the ability of clinical laboratories to respond quickly to future pandemic, chemical, and/or radiologic public health threats.
- The proposed rule would not be easily implementable, and it would create an insurmountable backlog of submissions that would hinder diagnostic innovation.
- The proposed rule limits the practice of laboratory medicine.
- The FDA has not evaluated less restrictive, easily administered alternatives, such as CLIA reform. This is particularly relevant for common test modifications used in most hospital and academic medical center settings.”
Here is the American Hospital Association:
…we strongly believe that the FDA should not apply its device regulations to hospital and health system LDTs. These tests are not devices; rather, they are diagnostic tools developed and used in the context of patient care. As such, regulating them using the device regulatory framework would have an unquestionably negative impact on patients’ access to essential testing. It would also disrupt medical innovation in a field demonstrating tremendous benefits to patients and providers.
Here is Mass General Brigham, a non-profit hospital system, affiliated with Harvard, and the largest hospital-based research enterprise in the United States:
…we are concerned with the heavy regulatory burden of this proposal. In implementing any regulatory structure, policymakers must consider if the benefits outweigh the costs. Given that FDA predicts 50 percent of tests would require premarket review, and 5 percent will require premarket approval, we have serious concerns that the costs may outweigh the benefits. Given that many LDTs are hospital-based and will never be commercialized, hospitals will have little incentive or ability to develop future LDTs under this proposed rule as they will have little to no opportunity to offset the costs associated with these new regulatory requirements. We are concerned that the regulatory burden could have significant implications on responsible innovation especially for LDTs targeting rare conditions, or public health emergencies.
Two U.S. public lab directors personally reached out to me to ask me amplify the warning. Consider it amplified!
Today is the last day for public comment. Get your comments in!
Selective reporting of placebo tests in top economics journals
Placebo tests, where a null result is used to support the validity of the research design, is common in economics. Such tests provide an incentive to underreport statistically significant tests, a form of reversed p-hacking. Based on a pre-registered analysis plan, we test for such underreporting in all papers meeting our inclusion criteria (n=377) published in 11 top economics journals between 2009-2021. If the null hypothesis is true in all tests, 2.5% of them should be statistically significant at the 5% level with an effect in the same direction as the main test (and 5% in total). The actual fraction of statistically significant placebo tests with an effect in the same direction is 1.29% (95% CI [0.83, 1.63]), and the overall fraction of statistically significant placebo tests is 3.10% (95% CI [2.2, 4.0]). Our results provide strong evidence of selective underreporting of statistically significant placebo tests in top economics journals.
That is from a new paper by Anna Dreber, Magnus Johannesson, and Yifan Yang.
Combination Rapid Tests
Once again, the US is behind on at-home rapid antigen tests–this time on combination tests that let you test for COVID, Influenza, and RSV all at once. These tests are widely available in Europe but have not been approved by the FDA. Rapid flu tests especially are potentially very useful in assigning appropriate treatment and reducing the overuse of antibiotics.
ProPublica on the FDA and Rapid Tests
Lydia DePillis has written the best piece on the FDA that I have ever read in a mainstream news publication. It gets everything right and yes it frankly verifies everything that I have been saying about the FDA and rapid tests for the last year and a half. I wish it had been written earlier but I suppose that illustrates how difficult it is to radically change people’s mindset from the FDA as protector to the FDA as threat. The sub head is:
Irene Bosch developed a quick, inexpensive COVID-19 test in early 2020. The Harvard-trained scientist already had a factory set up. But she was stymied by an FDA process experts say made no sense.
The piece recounts how cheap, rapid tests could have been approved in March of 2020! Here’s the opening bit:
When COVID-19 started sweeping across America in the spring of 2020, Irene Bosch knew she was in a unique position to help.
The Harvard-trained scientist had just developed quick, inexpensive tests for several tropical diseases, and her method could be adapted for the novel coronavirus. So Bosch and the company she had co-founded two years earlier seemed well-suited to address an enormous testing shortage.
E25Bio — named after the massive red brick building at MIT that houses the lab where Bosch worked — already had support from the National Institutes of Health, along with a consortium of investors led by MIT.
Within a few weeks, Bosch and her colleagues had a test that would detect coronavirus in 15 minutes and produce a red line on a little chemical strip. The factory where they were planning to make tests for dengue fever could quickly retool to produce at least 100,000 COVID-19 tests per week, she said, priced at less than $10 apiece, or cheaper at a higher scale.
“We are excited about what E25Bio is capable of shipping in a short amount of time: a test that is significantly cheaper, more affordable, and available at-home,” said firm founder Vinod Khosla. (Disclosure: Khosla’s daughter Anu Khosla is on ProPublica’s board.)
On March 21 — when the U.S. had recorded only a few hundred COVID-19 deaths — Bosch submitted the test for emergency authorization, a process the Food and Drug Administration uses to expedite tests and treatments.
You know how the story ends but really READ the WHOLE THING.
The Slow Rollout of Rapid Tests
I thought the Biden administration would at least make original pandemic errors. But no, its been making all the same errors. Slow on vaccines, slow on rapid testing and slow on new drugs, and far too little investment. Still after a year and half of shouting it from the rooftops we are getting some rapid tests. Josh Gans has an interesting reminder focusing on Canada that this has been an example of expert failure not just US failure.
Rapid test advocates such as myself have suddenly moved from fringe crazies who were told they didn’t understand the science to we need them and we need them now.
Several cases in point:
- The CDC now says that unvaccinated students exposed to Covid can “test to stay.” That is, rather than sending all the students in a class (or a school!) home when one tests positive for Covid, they test the students instead and so long as they are negative, they stay.
- The US Government is going to order 500 million rapid tests and distribute them free to the public … by mail!
It is hard to appreciate what a sea change this is in terms of attitude. A year ago, when we tried to roll out rapid tests — that had already been purchased and were sitting in their millions in warehouses in Canada — to Canadian workplaces, we were told that those tests had to be administered by health care professionals in PPE in secure and sanitised environments with all manner of precautions taken that really took the “rapid” out of rapid testing let alone exploding the costs to businesses who wanted to keep their workers safe. This was because they required those long-swabs etc. Eventually, short swabs were permitted. Then self-swabbing supervised in the workplace. Then swabbing at home while on a virtual call with a professional for that supervision with the swabs being picked up and then taken for safe disposal. Finally, we got to self-administered, at-home screening without supervision and you could pop your negative swan in the bin. A year after we had been told that you needed a full-court medical professional press to do this, our kids in Ontario were sent home with 5 rapid tests to use over the holidays. Only a couple of weeks ago, the Ontario government’s advisory board, the Ontario Science Table, finally endorsed the use of rapid tests in this way.
Update on Rapid Antigen Tests
In July of 2020 in Frequent, Fast and Cheap is Better than Sensitive, I wrote:
A number of firms have developed cheap, paper-strip tests for coronavirus that report results at-home in about 15 minutes but they have yet to be approved for use by the FDA because the FDA appears to be demanding that all tests reach accuracy levels similar to the PCR test. This is another deadly FDA mistake.
It’s depressing that we are still moving so slowly on these issues but the media has finally gotten on board. Earlier I mentioned David Leonhardt’s article. Here is Margaret Hartmann in the New York Magazine.
In many Asian and European countries, at-home COVID-19 tests are cheap and easy to find in stores. CBS News reported this month that home antigen tests are now used routinely in the U.K., where they are free and “readily available at pretty much every pharmacy in the country.”
The situation is drastically different here because U.S. health officials focused on getting people vaccinated against COVID-19 and never leaned into asymptomatic testing as a strategy to fight the pandemic. While some foreign governments moved quickly to encourage screening and subsidize the cost of at-home tests, the Food and Drug Administration’s approval process moved much more slowly.
….The FDA said it needed to ensure that the tests were accurate, but many scientists countered that the agency was letting the perfect be the enemy of the good.
Note also that this is a way of saying that the politicians have now also had it with the FDA:
In addition to ramping up production of tests already on the market, the government is also working to speed up the approval process. On October 4, the FDA authorized Flowflex, an at-home antigen test produced by ACON Laboratories that is expected to retail for around $10 per test. And on October 25, the Department of Health and Human Services announced that the FDA will streamline its authorization process, and the National Institutes of Health will spend $70 million on a new program to “establish an accelerated pathway” to aid test makers seeking approval for their products.
The NYTimes on the FDA and Rapid Tests
In July of 2020 I wrote in Frequent, Fast, and Cheap is Better than Sensitive:
A number of firms have developed cheap, paper-strip tests for coronavirus that report results at-home in about 15 minutes but they have yet to be approved for use by the FDA because the FDA appears to be demanding that all tests reach accuracy levels similar to the PCR test. This is another deadly FDA mistake.
…The PCR tests can discover virus at significantly lower concentration levels than the cheap tests but that extra sensitivity doesn’t matter much in practice. Why not? First, at the lowest levels that the PCR test can detect, the person tested probably isn’t infectious. The cheap test is better at telling whether you are infectious than whether you are infected but the former is what we need to know to open schools and workplaces.
It’s great that other people including the NYTimes are now understanding the problem. Here is the excellent David Leonhardt in Where are the Tests?
Other experts are also criticizing the Biden administration for its failure to expand rapid testing. Even as President Biden has followed a Covid policy much better aligned with scientific evidence than Donald Trump’s, Biden has not broken through some of the bureaucratic rigidity that has hampered the U.S. virus response.
In the case of rapid tests, the F.D.A. has loosened its rules somewhat over the past year, allowing the sale of some antigen tests (which often cost about $12 each). But drugstores, Amazon and other sellers have now largely run out of them. I tried to buy rapid tests this weekend and couldn’t find any.
The F.D.A.’s process for approving rapid tests is “onerous” and “inappropriate,” Daniel Oran and Dr. Eric Topol of Scripps Research wrote in Stat News.
For the most part, the F.D.A. still uses the same cumbersome process for approving Covid tests that it uses for high-tech medical devices. To survive that process, the rapid tests must demonstrate that they are nearly as sensitive as P.C.R. tests, which they are not.
But rapid tests do not need to be so sensitive to be effective, experts point out. P.C.R. tests often identify small amounts of the Covid virus in people who had been infected weeks earlier and are no longer contagious. Rapid tests can miss these cases while still identifying about 98 percent of cases in which a person is infectious, according to Dr. Michael Mina, a Harvard epidemiologist who has been advocating for more testing
Identifying anywhere close to 98 percent of infectious cases would sharply curb Covid’s spread. An analysis in the journal Science Advances found that test frequency matters more for reducing Covid cases than test sensitivity.
As I said on twitter what makes the FDA’s failure to approve more rapid antigen tests especially galling is that some of the tests being sold cheaply in Europe are American tests just ones not approved in the United States. If it’s good enough for the Germans it’s good enough for me!
Two brutal tests — can you pass them?
We all give people “tests” when we meet them, whether we are consciously aware of it or not. Here are two of mine:
1. The chess test. When I played chess in my youth, I would commonly analyze games with other players. You would then rapidly learn just how much and how quickly the other player could figure out the position and see imaginative variations. Some players maybe had equal or even inferior results to mine (I had a good work ethic and took no drugs), but it was obvious they were greater talents at analysis. Top chess players who worked with Bobby Fischer also attest that in this regard he was tops, not just “another great player.” That was true even before he was good enough and steady enough to become world champion.
When talking ideas with people, the same issue surfaces — just how quickly and how imaginatively do they grasp what is going on? You should put aside whatever they have or have not accomplished. How much do they have this Bobby Fischer-like capacity to analyze? No matter what their recent results have been (remember how Efim Geller used to kick Fischer’s butt in actual games?).
2. The art test. Take a person’s favorite genres of art, music, whatever. But something outside of their work lives. Maybe it can even be sports. How deeply do they understand the said subject matter? At what kind of level can they talk about it or enjoy it or maybe even practice it?
Remember in Hamlet, how Hamlet puts on a play right before the King’s eyes, to see how the King reacts to “art”?
Here we are testing for sensibility more than any kind of rigorous analysis, though the analysis test may kick in as well. Just how deep is the person’s deepest sensibility?
If you are investing in talent, you probably would prefer someone really good at one of these tests over someone who is “pretty good” at both of them.
3. All other tests.
Now, people can be very successful while failing both “the chess test” and “the art test.” In fact, most successful people fail both of these tests. Still, their kinds of success will be circumscribed. They are more likely to be hard-working, super-sharp, and accomplished, perhaps charismatic as well, while lacking depth and imaginative faculty in their work.
Nonetheless they will be super-focused on being successful.
I call this the success test.
Now if someone can pass the chess test, the art test, and the success test with flying colors…there are such people!
And if the person doesn’t pass any of those tests, they still might be just fine, but there will be a definite upper cap on their performance.
Update on Rapid Tests for COVID
Nearly a year ago, I wrote Frequent, Fast, and Cheap is Better than Sensitive, arguing for rapid antigen tests:
A number of firms have developed cheap, paper-strip tests for coronavirus that report results at-home in about 15 minutes but they have yet to be approved for use by the FDA because the FDA appears to be demanding that all tests reach accuracy levels similar to the PCR test. This is another deadly FDA mistake.
See also my posts Infected versus Infectious and Rapid Tests. The EMA and then the FDA finally did start approving these tests. So how well are they working? Pretty damn well. Canada has two innovative programs. First, in Nova Scotia pop-up clinics have been using rapid tests for asymptomatic people:
During the third wave that hit Nova Scotia over the past month, the province’s community rapid testing centres have correctly sniffed out at least 285 COVID-19 cases in asymptomatic people, or about 10 per cent of all confirmed cases in this time period, according to the Nova Scotia Health Authority.
While most provinces reserve testing only for symptomatic people or close contacts of a case, Nova Scotia’s pop-up centres allow asymptomatic people to simply show up and get a rapid test for free, with results sent to them within an hour. The whole process relies largely on volunteers without a health-care background.
Furthermore, the true number of cases credited to rapid testing is probably much higher. When a rapid test correctly identifies a positive case, the person’s close contacts such as their family get PCR lab tests that don’t show up in the rapid test statistics.
Lisa Barrett, an infectious diseases specialist and the driving force behind the rapid testing program, said it’s hard to say for certain, but taken altogether it’s possible rapid antigen testing has helped Nova Scotia find up to 18 per cent of all cases during the third wave.
“This is the early detection system,” Barrett said. Rapid testing tends to catch people early on in their infection when they’re full of virus, meaning positive cases are found and put into isolation fast — likely days before they would have been found with a PCR test, if they were found at all.
Michael Mina argues that since the rapid antigen detected cases are among the most infectious cases, detecting these cases is probably worth half of all the PCR testing.
Second, Canada’s CDL Rapid Screening Consortium is now in 200 sites with 50 large companies and rapidly expanding. A very interesting, just published paper in The Lancet runs an experiment that suggests that these testing regimes can work. The experiment rapidly tested 1000 people and the negatives were then randomly assigned either to be sent-home to conduct their regular life or to attend a multi-hour concert with masks but also singing, dancing, alcohol and no-social distancing. After 8 days there were two infections in the at-home group and no infections in the Concert group which suggests that this type of rapid testing can be used to open and keep-open concerts, schools, universities, airplanes and workplaces.
What’s the point of testing now that we have vaccines? Two reasons. First, most of the world still hasn’t been vaccinated so testing will be a very useful stop-gap measure until vaccination is more widely distributed. Indeed, the success of these programs shows what we lost by not acting more quickly a year ago. Second, although the pandemic is (essentially) over in the United States (as predicted) there will likely be an uptick in the fall among the unvaccinated and you want rapid tests to be available rapidly in hot-spots. In other words, rapid deployment of rapid tests will help us to avoid outbreaks in the future.
Rapid Antigen Tests in Canada
Josh Gans announces a program of Rapid Antigen Tests in Canada backed by a consortium of major Canadian companies.
Big News! Today I am very pleased to be able to reveal to the world something that I have been very proud to have been working on with a hundred or so other people: The CDL Rapid Screening Consortium. Led by our Creative Destruction Lab, this consortium is a group of 12 companies who are partnering with Health Canada to begin the roll-out of rapid antigen screens to be a part of daily life for the next 12-18 months and deliver a safer path to normality. We have been working since September intensively to put the consortium together, explore screening options that were available globally and come up with protocols and an evolving standard operating procedure (SOP) to bring rapid antigen screens at scale to economies all around the world. The goal is to solve the pandemic information gap and ensure that we can quickly identify and isolate infectious people and protect others.
The initial sites will be run by RSC members. Those members are Air Canada, Rogers, Loblaws, Shoppers Drug Mart, Magna, Nutrien, Suncor, Genpact, Scotiabank, MDA, CPPIB and MLSE.
Read Josh’s announcement for more details and here is the website for the CDL Rapid Screening Consortium.
Rapid Antigen Tests in Europe
Why are these tests important? The CDC now says that asymptomatic or pre-symptomatic people account for a majority of infections. Do you get it? How many people without symptoms will get a COVID PCR test, which can be time consuming and expensive? (And how many PCR tests can we run in a timely fashion if people without symptoms get many more tests?) Not that many. But many people without symptoms would get a $8 or less, at-home, 15 minute test. And if some of those people discover that they are infectious and self-isolate for a few days we can drive infection rates down.
We should have had an Operation Warp Speed for tests. We still need funding for a mass rollout and, of course, the FDA needs to approve these tests! (Here is Michael Mina in Time fulminating at the FDA holdup.)
By the way, more than 2800 Americans have died of COVID since Pfizer requested an Emergency Use Authorization for their vaccine. The FDA meets Dec. 10.
Addendum: Here’s me explaining why Frequent, Fast, and Cheap is Better than Sensitive and the difference between infected and infectious.
Rapid Antigen Tests
Rapid antigen tests are starting to be adopted worldwide.
Reuters: Germany, where infections jumped by 4,122 on Tuesday to 329,453 total, has secured 9 million so-called antigen tests per month that can deliver a result in minutes and cost about 5 euros ($5.90) each. That would, in theory, cover more than 10% of the population.
The United States and Canada are also buying millions of tests, as is Italy, whose recent tender for 5 million tests attracted offers from 35 companies.
Germany’s Robert Koch Institute (RKI) now recommends antigen tests to complement existing molecular PCR tests, which have become the standard for assessing active infections but which have also suffered shortages as the pandemic overwhelmed laboratories and outstripped manufacturers’ production capacity.
See my earlier posts Frequent, Fast, and Cheap is Better than Sensitive, Infected versus Infectious, and Rapid Tests for more on these types of tests.
Rapid Tests
Here’s a good picture illustrating the difference between the PCR and Rapid Test. A PCR amplifies DNA and so if taken at the right time it will detect the virus before a rapid test will. But this happens when there isn’t much viral load and too little of the virus to be transmissible. Moreover, at these times, the virus is increasing rapidly so the rapid test will find the virus tomorrow. The PCR test will also pick up fragments after transmissiblity has passed which also isn’t very useful. A rapid test is very sensitive for doing what it is supposed to do, identifying periods of infectiousness.
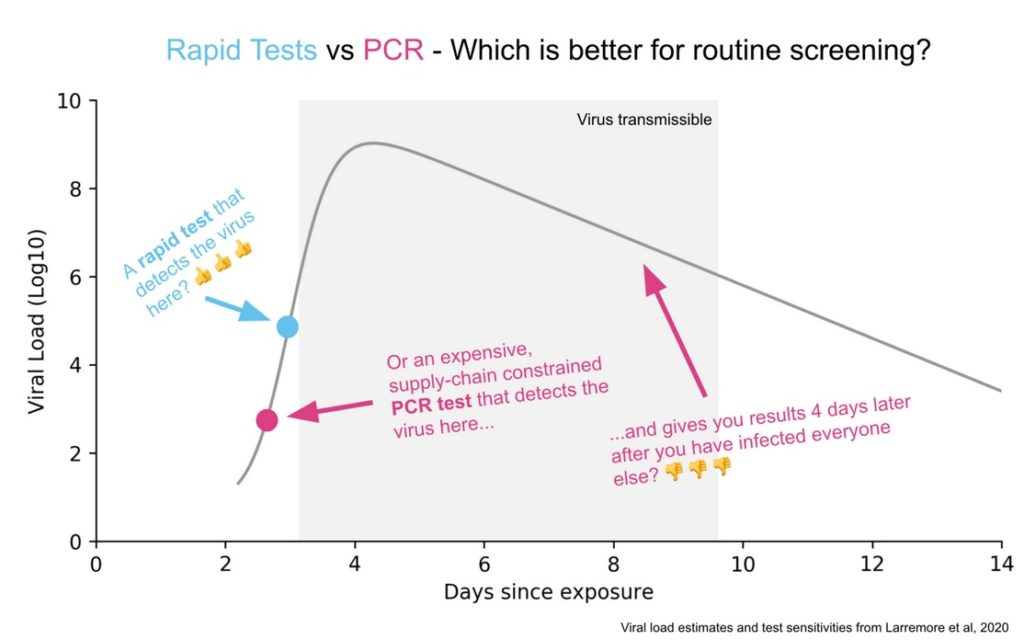
Michael Mina has done a great job promoting rapid tests and I do think we are beginning to see some recognition of the difference between infected versus infectious and the importance of testing for the latter. What is frustrating is how long it has taken to get this point across. Paul Romer made all the key points in March! (Tyler and myself have also been pushing this view for a long time).
In particular, back in March, Paul showed that frequent was much more important than sensitive and he was calling for millions of tests a day. At the time, he was discounted for supposedly not focusing enough on false negatives, even though he showed that false negatives don’t matter very much for infection control. People also claimed that millions of tests a day was impossible (Reagents!, Swabs!, Bottlenecks!) and they weren’t impressed when Paul responded ‘throw some soft drink money at the problem and the market will solve it!’. Paul, however, has turned out be correct. We don’t have these tests yet but it is now clear that there is no technological or economic barrier to millions of tests a day.
Go yell at your member of Congress.
FDA Allows Pooled Tests and a Call for Prizes
The FDA has announced they will no longer forbid pooled testing:
In order to preserve testing resources, many developers are interested in performing their testing using a technique of “pooling” samples. This technique allows a lab to mix several samples together in a “batch” or pooled sample and then test the pooled sample with a diagnostic test. For example, four samples may be tested together, using only the resources needed for a single test. If the pooled sample is negative, it can be deduced that all patients were negative. If the pooled sample comes back positive, then each sample needs to be tested individually to find out which was positive.
…Today, the FDA is taking another step forward by updating templates for test developers that outline the validation expectations for these testing options to help facilitate the preparation, submission, and authorization under an Emergency Use Authorization (EUA).
This is good and will increase the effective number of tests by at least a factor of 2-3 and perhaps more.
In other news, Representative Beyer (D-VA), Representative Gonzalez (R-OH) and Paul Romer have an op-ed calling for more prizes for testing:
Offering a federal prize solves a critical part of that problem: laboratories lack the incentive and the funds for research and development of a rapid diagnostic test that will, in the best-case scenario, be rendered virtually unnecessary in a year.
…We believe in the ability of the American scientific community and economy to respond to the challenge presented by the coronavirus. Congress just has to give them the incentive.
The National Institutes of Health (NIH) have already begun a similar strategy with their $1.4 billion “shark tank,” awarding speedy regulatory approval to five companies that can produce these tests. Expanding the concept to academic labs through a National Institute of Science and Technology (NIST)-sponsored competition has the added benefit ultimately funding more groundbreaking research once the prize money has been awarded.
This is all good but frustrating. I made the case for prizes in Grand Innovation Prizes for Pandemics in March and Tyler and I have been pushing for pooled testing since late March. We were by no means the first to promote these ideas. I am grateful things are happening and relative to normal procedure I know this is fast but in pandemic time it is molasses slow.