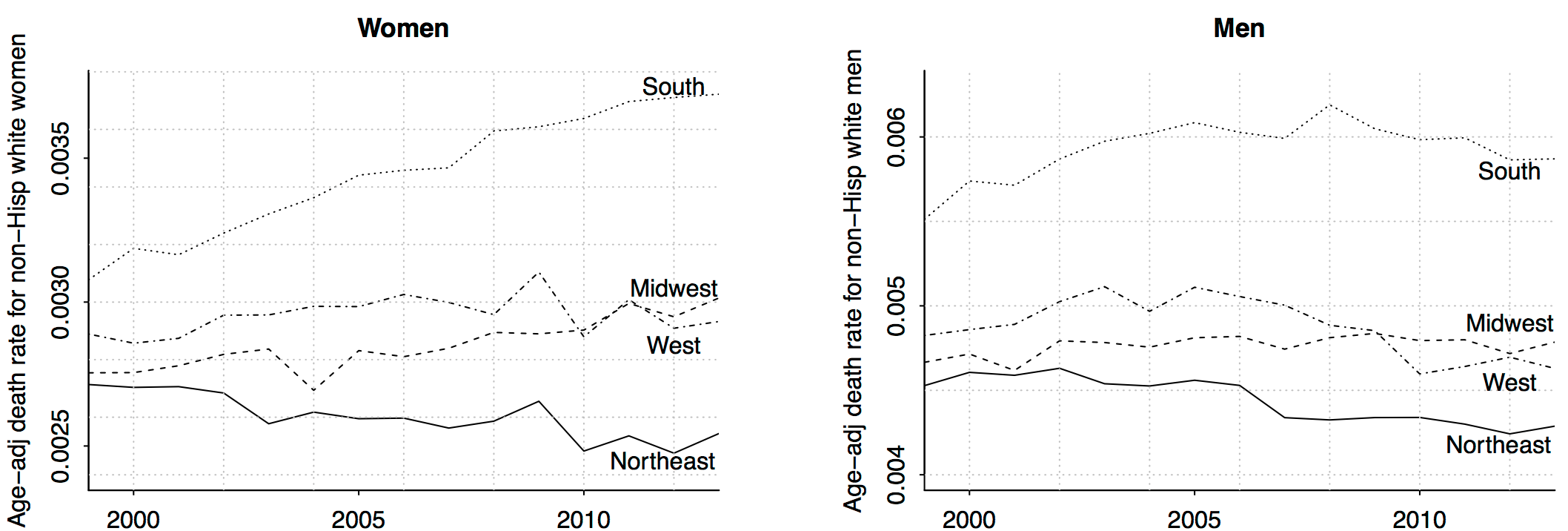
Category: Medicine
It’s all about women in the South
Wow!! Remember that increasing death rate among middle-aged non-Hispanic whites? It’s all about women in the south (and, to a lesser extent, women in the midwest). Amazing what can be learned just by slicing data.
I don’t have any explanations for this. As I told a reporter the other day, I believe in the division of labor: I try to figure out what’s happening, and I’ll let other people explain why.
That is from Andrew Gelman, there is more at the link.
Economists on FDA Reciprocity
Daniel Klein & William Davis surveyed economists about whether it would be an improvement to reform the FDA so that “as soon as a new drug is approved by any one of five [FDA approved international] agencies, that drug automatically gains approval in the United States.” They report:
Of the 467 economists who answered the question and did not mark “Have no opinion,” 53 percent agreed that the reform would be an improvement, while 29 percent disagreed. (The remainder said they were “neutral.”) Moreover, those favoring the reform were more likely to say they held their belief “strongly.” Hence, the balance of economist judgment certainly leaned in favor of the liberalization.
Economists are not the only ones in favor of reciprocity. Others are also coming around, at least partially. In Generic Drug Regulation and Pharmaceutical Price-Jacking I argued in response to the massive increases in the price of Daraprim (generic name Pyrimethamine) that we ought to allow importation:
Pyrimethamine is also widely available in Europe. I’ve long argued for reciprocity, if a drug is approved in Europe it ought to be approved here. In this case, the logic is absurdly strong. The drug is already approved here! All that we would be doing is allowing import of any generic approved as such in Europe to be sold in the United States.
In a paper in JAMA discussing the same case, Drs Jeremy Greene, Gerard Anderson, and Joshua M. Sharfstein agree, writing:
A second option is to temporarily permit the importation of drug products reviewed by competent regulatory authorities and approved for sale outside the United States. For example, Glaxo, the original manufacturer of pyrimethamine, sells a version of the drug approved for use in the United Kingdom at less than $1 per tablet.
Dr Sharfstein by the way was Principal Deputy Commissioner of the US Food and Drug Administration from March 2009 to January 2011.
Addendum: I will be discussing/debating pharmaceutical policy with Dr. Sharfstein at on event sponsored by the Council on Foreign Relations in Washington, DC the morning of Monday January 25. Invitation only but email me if you want an invite.
Do you get grumpy when it is warmer than seventy degrees Fahrenheit?
I don’t think climate change is the right framing for this effect, nonetheless this is an interesting result, with the subtitle “Evidence from a billion tweets.” Here is the abstract:
What is the welfare cost of environmental stress? The change in amenity values resulting from temperature increases may be a substantial unaccounted-for cost of climate change. Because there is no explicit market for climate, prior work has relied on cross-sectional variation or survey data to identify this cost. This paper presents an alternative method of estimating preferences over nonmarket goods which accounts for unobserved cross-sectional and temporal variation and allows for precise estimates of nonlinear effects. Specifically, I create a rich dataset on hedonic state: a geographically and temporally dense collection of updates from the social media platform Twitter, scored using a set of both human- and machine-trained sentiment analysis algorithms. Using this dataset, I find limited evidence of temperature effects on hedonic state in low temperatures and strong evidence of a sharp decline in hedonic state above 70◦F. This finding is robust across all measures of hedonic state and to a variety of specifications.
That is the job market paper (pdf) by Patrick Baylis, a job candidate from UC Berkeley.
And here is a new result that Canadians are more polite on Twitter, I wonder what happens if you control for temperature…
For the pointer I thank Samir Varma.
Are the disabled less loss averse?
Arbel, Ben-Shahar, and Gabriel have a newly published paper on this topic, here is the abstract:
Research findings show that disabled persons often develop physical and psychological mechanisms to compensate for disabilities. Coping mechanisms may not be limited to the psychophysiological domain and may extend to cognitive bias and loss aversion. In this study, we apply unique microdata from a natural policy experiment to assess the role of loss aversion in home purchase among nondisabled and disabled households. Results of survival analysis indicate that the physically disabled are substantially less loss averse in home purchase. Furthermore, loss aversion varies with other population characteristics and attenuates with degree of disability. Findings provide new evidence of diminished cognitive bias and more rational economic decision-making among the physically disabled.
There are alternative versions of the paper here.
For the pointer I thank the excellent Kevin Lewis.
Obamacare in 2016
During the election season Democrats can’t admit Obamacare is broken and Republicans can’t admit it won’t be repealed.
An excellent post from Robert Laszewski, read the whole thing.
Death trends
The Omnibus spending bill on health care policy
Yuval Levin has a very good piece on this, here is one bit:
They’re [the Democrats] no longer offering themselves up as a sacrifice to protect every last bit of the law[Obamacare], as they have done at enormous political cost for the last five years. Now, they’re spending their capital to protect key constituencies (and therefore themselves), even at the cost of allowing the structure of Obamacare to become even more incoherent and unsustainable.
There is a less polemic but still true version of that sentence, if you are so inclined. Here is another bit:
…They’re thinking past Obamacare, like the Republicans are. Of course, Democrats have a different vision of what comes after Obamacare. Hillary Clinton has started articulating that vision here and there: It’s a move in the direction of the original Hillarycare from 1993, which would add on to elements of Obamacare stricter price controls and more federal micromanagement of the provision of care. (Scott Gottlieb considered what this might look like in National Affairs this summer.)
And this:
The omnibus bill contains a provision, identical to one in last year’s bill, which requires that risk-corridor payments in the Obamacare exchanges be budget neutral.
That will make Obamacare much more difficult to manage.
Furthermore, in the bill Congress restricts federal funding for CRISPR.
Here is a more general piece on the Omnibus. Overall I would say a lot of gridlock is gone, the Republicans have returned to being bigger spenders, and no one in town — once again — worries about the deficit. The sequester was a very temporary victory.
Genetic testing may be coming to your office
A handful of firms are offering employees free or subsidized tests for genetic markers associated with metabolism, weight gain and overeating, while companies such as Visa Inc., Slack Technologies Inc., Instacart Inc. recently began offering workers subsidized tests for genetic mutations linked to breast and ovarian cancer.
The programs provide employees with potentially life-saving information and offer counseling and coaching to prevent health problems down the road, benefits managers say.
Screening for genetic markers linked to obesity is the latest front in companies’ war on workers’ weight woes.
Obesity-related conditions such as Type 2 diabetes comprise a large share of overall health-care costs, estimated to run more than $12,000 a worker this year, according to a recent survey from Towers Watson and the National Business Group on Health.
Employers are hoping to help bend the cost curve—and make their workers healthier—by more aggressively targeting obesity and coaxing workers to lose weight.
Fortunately, none of that information ever will be used against the interests of workers, nor will any worker face pressure, explicit or implicit, to submit to such a test…
The story is here, here is another path in.
Very good New York Times sentences Arlington fact of the day
“Consider Arlington, Va., our best guess for where you might be reading this article.”
That is from an excellent NYT piece on health care and prices. The very interesting original research is here (pdf), main point is that where (properly adjusted) Medicare spending is high is surprisingly uncorrelated with where private health care spending is high. Furthermore policy may have been encouraging too many hospital mergers.
Here is Kevin Drum summarizing the study’s results on the importance of competition.
The FDA and Magical Thinking
Vox had a piece yesterday on the Cruz-Lee proposal to make it easier for U.S. patients to access drugs and devices already approved in other developed countries. The Vox piece had some howlers. Most notably this:
“There’s no evidence the FDA blocks innovation or makes innovation harder or makes it more costly,” said Kesselheim.
Frankly, that would be laughable were it not coming from a professor of medicine at Harvard Medical School. It costs well over a billion dollars to get the average new drug approved and much of that cost comes from FDA required clinical trials. Longer and larger clinical trials mean that the drugs that are eventually approved are safer. But longer trials also mean that good drugs are delayed. And the more expensive it is to produce new drugs the fewer new drugs will be produced. In short, longer and larger trials mean drug delay and drug loss.
We live in a world of tradeoffs. Let’s debate the tradeoffs. But let’s not engage in magical thinking where there are no tradeoffs and “no evidence” that the FDA makes drug development more costly.
A more subtle error was committed by the author who writes:
But it’s not clear that this legislation can solve the biggest problem here — the lack of promising treatments in the pipeline. In other words, a faster approval process can’t fix a dearth of innovation from labs themselves.
Many factors go into drug development that are outside the FDA’s purview. Nevertheless, faster drug approval can and does increase innovation. Approving drugs more quickly is equivalent to a decrease in the costs of research and development. Time is money. Reducing the cost of development increases the incentive to develop new drugs.
The Prescription Drug User Fee Act, for example, reduced drug approval times by about 10 months. Philipson et al. calculate that:
…the more rapid access of drugs on the market enabled by PDUFA saved the equivalent of 140,000 to 310,000 life years.
(PDUFA does not appear to have materially affected safety but Philipson et al. calculate that even under a worst case scenario the benefits of PDUFDA far exceeded the costs).
Moreover, Vernon et al. find that the reduction in approval time from PDUFA increased new drug development:
Controlling for other factors such as pharmaceutical profitability and cash flows, we estimate that a 10% decrease (increase) in FDA approval times leads to an increase (decrease) in R&D spending from between 1.4% and 2.0%. Combining this estimate with recent research on the link between PDUFA and FDA approval times…we calculate PDUFA may have incentivized an additional $10.8 billion to $15.4 billion in pharmaceutical R&D. Recent economic research has shown that the social rate of return on pharmaceutical R&D is very high; therefore, the social benefits of PDUFA (over and above the benefits of more rapid consumer access) are likely to be substantial.
Finally, return to the issue of reciprocity. Many of the critics of reciprocity respond with simple appeals to nationalism. We are the best! Rah, rah, rah! But if the critics were German or French they would argue that the EMA is superior to the FDA. Indeed, when I raise the issue of reciprocity with Europeans they respond in exactly the same way as Americans. How could anyone suggest that the EMA automatically approve drugs approved by the FDA! The horror.
The argument for reciprocity, however, isn’t that the FDA is uniquely bad or always worse than the EMA or vice-versa. The argument is that it’s wasteful to duplicate the lengthy approval process and that both agencies sometimes make mistakes. As a result, it’s simple common sense to let Americans avail themselves of drugs and devices approved in other developed countries.
Senators Cruz and Lee Introduce Reciprocity Bill
Senators Ted Cruz (R-Texas) and Mike Lee (R-Utah) have just introduced a bill that would implement an idea that I have long championed, making drugs, devices and biologics that are approved in other developed countries also approved for sale in the United States. Highlights of the “Reciprocity Ensures Streamlined Use of Lifesaving Treatments Act (S. 2388), or the RESULT Act,” include:
- Amending the Food, Drug and Cosmetic Act to allow for reciprocal approval of drugs, devices and biologics from foreign sponsors in certain trusted, developed countries including EU member countries, Israel, Australia, Canada and Japan.
- Encouraging the FDA to expeditiously review life-saving drug and device applications, this legislation would provide the FDA with a 30-day window to approve or deny a sponsor’s application….
- The HHS Secretary is instructed to approve a drug, device or biologic if the FDA confirms the product is:
- Lawfully approved for sale in one of the listed countries;
- Not a banned device by current FDA standards;
- There is a public health or unmet medical need for the product.
- If a promising application for a life-saving drug is declined Congress is granted the authority to disapprove of a denied application and override an FDA decision with a majority vote via a joint resolution.
In explaining why he introduced the bill Senator Cruz argued:
We continue to lose far too many of our loved ones to the “invisible graveyard,” as economist Alex Tabarrok has described: lives that could have been saved but for a bureaucratic barrier that rejects medical cures and innovation…The bill I am introducing takes the first step to reverse this trend. It provides for reciprocal drug approval, so that cures and medical devices that are already approved in other countries can more expeditiously come to the U.S.
The marginal value of health care and hospital admission
Here is the job market paper of Nathan Petek, from the Booth School of Business, University of Chicago:
Abstract: The marginal benefit of health care determines the extent to which policies that change health care consumption affect health. I use variation in access to hospitals caused by nearly 1,300 hospital entries and exits to estimate the marginal benefit of inpatient care. I show that hospital entries and exits cause sharp changes in the quantity of inpatient care, but there is no evidence of an effect on average mortality with tight confidence intervals. I find suggestive evidence of an effect on mortality in rural areas and for the over-65 population with magnitudes that imply the marginal benefit of inpatient care is significantly higher for these populations than for the average patient.
Even for rural areas and the elderly, an effect is not seen until more than a year after the event.
By the way, $900 billion is spent annually at U.S. hospitals.
For the pointer I thank David, a loyal MR reader.
Why medical progress is difficult
Here is part of the abstract of a new NBER paper from Gisela Hostenkamp and Frank R. Lichtenberg:
We use Danish diabetes registry and health insurance data to analyze the extent, consequences, and determinants of under-use and overuse of oral anti-diabetic drugs.
Less than half of patients consume the appropriate amount of medication–between 90% and 110% of the amount prescribed by their doctors.
The life expectancy of patients consuming the appropriate amount is 2.5 years greater than that of patients consuming less than 70% of the prescribed amount, and 3.2 years greater than that of patients consuming more than 130% of the prescribed amount, controlling for time since diagnosis, insulin dependence, comorbidities, age, gender and education. Patients consuming the appropriate amount are also less likely to be hospitalized than under- or over-users.
And of course that is from Denmark, which is supposed to be a culture with relatively strong norms of conscientiousness.
China fact (estimate) of the day
The China Medical Doctors’ Association recently found that 13 per cent of doctors surveyed had been physically attacked in the past year.
The FT article by Andrew Ward and Patti Waldmeir is interesting throughout, here is another bit:
Doctors are paid on average one-fifth the amount received by their counterparts in Europe and are required to see up to 150 patients a day.
Overall the Chinese health care system is considered to be extremely corrupt.
The optimal regulation of massage and prostitution
The job market paper of Amanda Nguyen, of UCLA, is on that topic, I found her results intriguing:
Despite its illegality, prostitution is a multi-billion dollar industry in the U.S. A growing share of this black market operates covertly behind massage parlor fronts. This paper examines how changes to licensing in the legal market for massage parlors can impact the total size and risk composition of the black market for prostitution, which operates either illegally through escorts or quasi-legally in massage parlors. These changes in market structure and risk consequently determine the net impact of prostitution on sexually transmitted diseases (STDs) and sexual violence. I track the impact of two policy changes in California that resulted in large variation in barriers to entry via massage licensing fees. Using a novel dataset scraped from Internet review websites, I find that lower barriers to entry for massage parlors makes the black market for prostitution larger, but also less risky. This is due to illegal prostitution buyers and suppliers switching to the quasi-legal sector, as well as quasi-legal sex workers facing a reduced wage premium for high-risk behavior. Consequently, the incidence of gonorrhea and rape falls in the general population. I also present evidence that growth in the quasi-legal sector imposes a negative competition externality on purely legal massage firms.
I don’t find the rape result intuitive, but I am seeing it pop up in a number of papers, so perhaps it should be taken seriously.