Category: Medicine
Vaccine access toolkit
In order to break down the information barrier, I partnered with my local faith community to set up a vaccine outreach program. We have a dedicated vaccine information email address and a website consolidating information about vaccine eligibility, sites, and benefits. We call community members who don’t have email, offer personal assistance making appointments and connecting to ride services, and provide information about best practices for riding safely in a car with masks and open windows. After vaccine appointments, we check in to see how they are feeling, make sure their second dose is scheduled, and offer to drop off comfort foods. One recipient replied, “It makes this daunting time just a little easier knowing there is someone out there to guide one through this process. My technology skills are minimal!”
By Margaret Scharle, for Oregon. We need much more of this.
Osterholm on First Doses First
Here from a podcast is Michael Osterholm, Regents Professor, McKnight Presidential Endowed Chair in Public Health, the director of the Center for Infectious Disease Research and Policy (CIDRAP) and state epidemiologist for the Minnesota Department of Health.
…Imagine you are setting across the table from two people both of whom are 65 or older, both with underlying health conditions. You have two doses of vaccine, one in each hand. And you say to them I can give two doses to you or to you but then the other person gets nothing. Or I can give one dose to both of you. And this is what I know. At the very least, one dose is likely to prevent serious illness, hospitalization and death. Two doses will probably even prevent clinical disease with B.1.1.7. But the other one of you; if you get infected with this virus, which I think substantial numbers of Americans will, things are not looking good for you. What do you want me to do?
If that is your Mom or Dad. Your Grandpa or Grandma. What would you do?
This is where the rubber meets the road. I think if the data bears it out we can save so many lives in the upcoming weeks and we are missing that opportunity.
I have already made my choice. I am postponing my second dose. I want my second dose. But I am confident that I can wait. And I can only hope that my second dose, which I have just deferred, will go to someone who it will save their life. It will make a totally different world for that family.
You know some could argue that this could be the end of my career. But I could not sleep with myself at night if I didn’t do this. I just know in my heart of hearts that this is something we must do if we are going to save lives.
The entire podcast is worthwhile, this is from around minute 44:30 (my imperfect transcription).
Hat tip: Anon.
Addendum: Many other countries should be looking very closely at dose-stretching policies.
In other news, South Korea approves the AstraZeneca vaccine. It’s not like we have anything to learn from South Korea about managing a pandemic, right? Right?
How much do we value Covid safety?
The grand experiment of blocking the middle seat on airplanes has proved what we have known all along about air travel: More people care about a cheap fare than comfort, or even pandemic safety.
Delta announced on Monday that it was extending its middle-seat block for one more month, to the end of April. Delta, the last U.S. airline to block all middle seats in coach, will consider further extensions based on Covid-19 transmission and vaccination rates.
So far, Delta thinks it’s earning goodwill and confidence with customers, particularly business travelers, who aren’t traveling now but will come back. Some who’ve flown during the pandemic have been willing to pay Delta more for more space onboard. Most have been price-sensitive leisure travelers willing to sit shoulder-to-shoulder for cheap fares—on airlines not blocking middle seats…
The bottom line for Delta during the pandemic has been bigger losses than rival airlines selling all their seats. Delta was the most profitable U.S. airline in the final six months of 2019. That flipped during the pandemic. In the last six months of 2020, Delta had the biggest losses, with a net loss of more than $6 billion, greater than United and Southwest combined.
Mr. Lentsch says Delta can’t keep blocking middle seats forever.
Here is more from the WSJ. I do get there is an externality here, so people are not paying enough for those more spacious Delta seats, as they do not take their higher risk to others into sufficient account. Still, a lot of the risk here is private, and I feel the public health community in the United States has not been willing to look such data in the face squarely enough. Is the public policy problem about minimizing “lives lost,” or maximizing “welfare,” or giving people “what they want”? Or some combination of those? Who exactly has been good at thinking through those trade-offs?
Have the pandemic population flows been into the relatively strict Vermont and California, or to the relatively open Florida and Texas?
To what extent is the real externality a kind of degradation of the public sphere, and the spread of stress and mental health problems, rather than the health of others per se?
Worth a ponder.
Canada Needs a New Vaccination Strategy
The US vaccination rollout has been deadly slow, inefficient, and chaotic. It has also been one of the best in the world. Canada, for example, is far behind the US on vaccination.
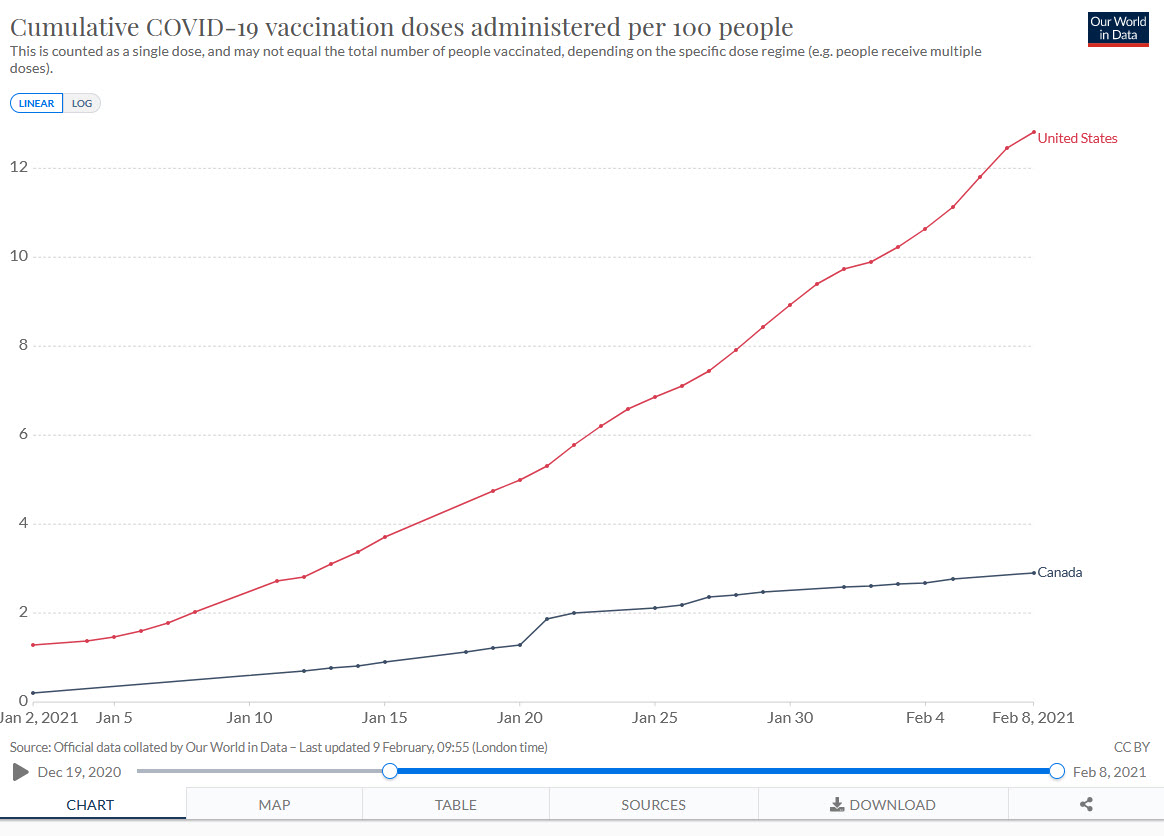
The Canadian deficit is mostly because they don’t have enough vaccine. Canada bought doses but they didn’t invest in capacity and a deal with China fell through. As a result, Canada won’t be getting lots of vaccine until March or April. Operation Warp Speed invested billions in the Modern vaccine and in early purchases of the Pfizer vaccine and thus got first dibs. The Americans are also not allowing vaccine to be exported to Canada. (We could at least give them access to our AstraZeneca factory!).
Regardless of blame, this puts Canada in a precarious situation. Death rates aren’t as high as in the United States but with new variants exploding, Canada is running a big risk. To get Canadians vaccinated more quickly–including my mother–Canada needs to find ways to stretch their vaccine supply–that means First Doses First, half dosing, intradermal delivery and other dose stretching strategies should be considered.
Many other countries are in a much more worse position than either the United States or Canada.
One Shot if You Have Been Infected
Here’s noted microbiologist Florian Krammer:
This is now the third paper to find a very very good immune response after one shot of mRNA vaccine in people who had a previous SARS-CoV-2 infection. Time to discuss policy changes, @DrNancyM_CDC@CDCDirector
Paper here.
In other words, as I wrote earlier, “for the 25 million to 100 million Americans who have already been infected by COVID it may be better for them personally to delay the second dose….[thus] a significant fraction of second doses have little to no value.”
It’s good that people like Krammer are signaling that it’s time for policy change. Still, I am frustrated. None of this is unexpected or surprising. It’s just that some people (n.b. I am not referring to Krammer in particular) do not have the training or the mindset to make cost-benefit decisions under uncertainty. That’s ok in ordinary times but during a war, pandemic or takeover fight it’s deadly.
Who first noticed the tech was ready for human genome sequencing? (that was then, this is now)
Perkin Elmer’s last purchase had been a Cambridge, Massachusetts, company called PerSeptive Biosystems, a protein-analysis enterprise started by Lebanese-born wunderkind Noubar Afeyan seven years earlier, when the ink was still wet on his Ph.D. from MIT. Afeyan had sold his company to Perkin Elmer for almost $400 million. The deal had yet to be finalized, and formally speaking Afeyan wasn’t yet a Perkin Elmer employee. But he was at the meeting in Foster City, too. Like Lipe and Barrett, he shared White’s vision of “moving up the food chain” and getting into the genetic information business. But like them, he didn’t really have a clear idea how that was to be done.
It was Afeyan, the newcomer, who first said the words. The head of the multicapillary-machine production team was winding up a presentation on the instrument’s design and capacity. There were twenty-odd people around the table, discussing such matters as pricing, costs, and marketing strategy. This was the first time Afeyan had heard that the project even existed. He was taking some notes and idly doing some calculations. “You know, with enough of these machines, we could sequence the whole human genome,” he remarked. A few people chuckled at the notion, and the discussion returned to serious topics. But now Hunkapiller was hunched over his yellow pad, scribbling. After a minute he looked up. “He’s right,” he said. “Who’s right?” asked White. “Noubar. With two hundred machines, we could sequence the human genome in three years.” Most people in the room hardly knew Noubar Afeyan, but they knew Michael Hunkapiller. He would not interrupt a serious discussion except for something even more serious.
That is from James Shreeve’s The Genome War, here is more detail from the NYT in 1999, and for the pointer I thank Patrick Collison. Of course that is the same Afeyan Noubar who co-founded Moderna and now chairs it, here is my earlier CWT with him.
How to Double the Number of Moderna and Pfizer Factories
Theory and data both suggest that a much smaller dose–perhaps as low as 1/4 the current dose—of both the Moderna and Pfizer vaccines are as effective as a larger dose. Half doses of Moderna and Pfizer would be equivalent to instantly doubling the number of Moderna and Pfizer factories and would save hundreds of thousands of lives and be worth hundreds of billions of dollars in world GDP. Clinical data from adults 18-55 from the Moderna Phase II trial already suggest that quarter-doses are effective, which is why Operation Warp Speed chief, Moncef Slaoui has advocated for half-doses (leaving plenty of margin).
“We know that, for the Moderna vaccine, giving half of the dose to people between the ages of 18 and 55, two doses [at] half the dose … we know it induces identical immune response” to the currently authorized dose, Slaoui added.
Another way of putting this is that new clinical trials on dosing would be tremendously valuable. Ideally, we could use correlates of protection and do a bridging trial to infer the effectiveness of half-doses. The FDA has already said they will accept a bridging trial for new mRNA vaccines for variants, which is the right decision. The FDA should also accept a bridging trial for new dosing.
If new clinical trials are deemed necessary, dosing trials could be run as challenge trials but instead of comparing half-doses to placebo we would compare full-doses to half-doses. Thus, everyone in the challenge trial would be vaccinated, massively lowering risks. If we can’t do challenge trials even with vaccinated volunteers (!) then let’s get started on clinical trials. The NIH created the ACTIV program to speed clinical trials. Use it.
New clinical trials are valuable not just for dosing but also for approving new vaccines. Equivalence trials on the Sputnik and Sinopharm vaccines, for example, could be very valuable. In other words, we would trial Sputnik and Sinopharm against Pfizer and Moderna. A lot of people would be quite willing to join a trial in which the worst outcome is most likely getting a somewhat less effective vaccine–that’s much better than no vaccine!
The value of experiments, or let’s call them pilot studies, right now is immense. We can do pilot studies on half dosing for Moderna and Pfizer vaccines much faster and cheaper than we can build twice as many factories. So let’s do it!
Emmott Interviews Tabarrok for the Browser
Bill Emmott, former editor-in-chief of The Economist and now co-director of the Global Commission for Post-Pandemic Policy, talks to Alex Tabarrok, Professor of Economics at George Mason University and co-author of the blog Marginal Revolution, on lessons learned from the pandemic so far, and what lies ahead.
Self-recommending. I’d say it’s a very good interview but there was no question that I was outclassed by Bill Emmott’s zoom background, live from Dublin. Many thanks to the ever-excellent The Browser for hosting.
https://youtu.be/0FBjW6KqyTU
First Doses First, Now! – New Information
A lot of new information has dropped recently about the efficacy of First Doses First.
First, as I mentioned yesterday, we now have epidemiologists and vaccine researchers saying that for people previously infected with COVID a second dose is not necessary and may be “overkill.” Given how many people have had COVID, this increases the net benefit to First Doses First for everyone significantly.
Second, an important new study verifies that for the AZ vaccine a longer delay for the second dose is better because it generates a more powerful immune response (picture from the FT). This  is a common finding for vaccines. The authors write:
is a common finding for vaccines. The authors write:
ChAdOx1 nCoV-19 vaccination programmes aimed at vaccinating a large proportion of the population with a single dose, with a second dose given after a 3 month period is an effective strategy for reducing disease, and may be the optimal for rollout of a pandemic vaccine when supplies are limited in the short term.
In addition “Analyses of PCR positive swabs in UK population suggests vaccine may have substantial effect on transmission of the virus with 67% reduction in positive swabs among those vaccinated.” In other words, the vaccine cuts transmission risk.
As I have said before “the US failure to authorize the AstraZeneca vaccine in the midst of a pandemic when thousands are dying daily and a factory in Baltimore is warmed up and ready to run is a tragedy and dereliction of duty of epic proportions.”
Third, the New and Emerging Respiratory Virus Threats Advisory Group (NERVTAG), a scientific advisory group to the British government, recently considered the risks of immune escape from the delayed second dose strategy and concluded that although the risk is real it is likely small, especially in comparison to other sources of immune escape such as therapeutics and natural infection. Moreover, the risk is outweighed by the measurable benefits of getting more does out quickly.
It is not currently possible to quantify the probability of emergence of vaccine resistance as a result of the delayed second dose, but it is likely to be small.The UK currently has more than 1,000 COVID-19 related deaths each day and has limited supplies of vaccine. In the current UK circumstances the unquantifiable but likely small probability of the delayed second dose generating a vaccine escape mutant must be weighed against the measurable benefits of doubling the speed with which the most vulnerable can be given vaccine-induced protection.
..a single dose of vaccine does not generate a new/novel risk. Given what we have observed recently with the variants B.1.1.7 and B1.351, it is a realistic possibility that over time immune escape variants will emerge, most likely driven by increasing population immunity following natural infection.
Fourth, the British health establishment has largely solidified around First Doses First. Consider this from the Four UK’s Chief Medical Officers.
The 4 UK Chief Medical Officers agree with the JCVI that at this stage of the pandemic prioritising the first doses of vaccine for as many people as possible on the priority list will protect the greatest number of at risk people overall in the shortest possible time and will have the greatest impact on reducing mortality, severe disease and hospitalisations and in protecting the NHS and equivalent health services.
Fifth, the US public health experts are beginning to come around to the economic point of view. Consider Experts tout delaying 2nd COVID vaccine dose as US deaths mount which notes:
“The maximum public health benefit would come from giving a single dose to as many people as possible, and following up with a second dose when supply improves,” said Neal Halsey, MD, of Johns Hopkins University, in an interview. Halsey and Stanley Plotkin, MD, co-authored a letter in Clinical Infectious Diseases last week explaining how delaying a second dose of vaccine would accelerate the US vaccine rollout.
Halsey said data from both companies show the first dose of the vaccine offers significant protection against COVID-19 in the short term, for at least 1 to 3 months after injection. He also said he and Plotkin believe this was the most beneficial public health strategy even before the arrival of new variants of the virus was discovered.
“There are a number of examples of changing [vaccination] course because ACIP takes into account public health impact,” Halsey said. “We asked the ACIP to review in depth this strategy to give one dose as rapidly as possible. Such a meeting should be scheduled as soon as possible.”
The University of Minnesota’s Michael Osterholm, PhD, MPH, said yesterday on “Meet the Press” that he believes the United States has to change direction on vaccine strategy in light of the possibility of a surge of new infections coming from variant strains.
I believe that the US will go to First Doses First. The only question is will we go to First Doses First soon, when it can still help, or will we be forced to do it later in an act of desperation and agony.
Had Covid? You May Need Only One Dose
The barriers are breaking. Step by step we move closer to First Doses First. New results from a small-scale study suggest that people who have had COVID have strong reactions to the first dose and may not need the second dose.
NYTimes: Based on these results, the researchers say, people who have had Covid-19 may need only one shot.
“I think one vaccination should be sufficient,” said Florian Krammer, a virologist at the Icahn School of Medicine at Mount Sinai and an author on the study. “This would also spare individuals from unnecessary pain when getting the second dose and it would free up additional vaccine doses.”
…People who have had Covid seem to be “reacting to the first dose as if it was a second dose,” said Akiko Iwasaki, an immunologist at the Yale School of Medicine. So one dose is probably “more than enough,” she said.
A study published earlier this month reported that surviving a natural infection provided 83 percent protection from getting infected again over the course of five months. “Giving two doses on top of that appears to be maybe overkill,” she added.
So for the 25 million to 100 million Americans who have already been infected by COVID it may be better for them personally to delay the second dose. In short, a significant fraction of second doses have little to no value. This (unsurprising) finding means that First Doses First is an even better strategy even if we can’t condition doses on previous infection.
Most important, First Doses First gets more people significant immunity faster which is good for the vaccinated and also drives down R which is good for society as a whole, even the unvaccinated.
The Biden administration has been more pro-active than the Trump administration on tests and vaccination and has already made some goods calls on getting more doses out faster. I hope they continue to be bold. We need quick, bold, and decisive action.
Just A Few Weeks Delay
NYTimes: Federal regulators could decide within a few weeks whether to allow Moderna, the Massachusetts biotech company that developed one of the two federally authorized Covid-19 vaccines, to increase the number of doses in its vials — which could accelerate the nation’s vaccination rate.
Moderna is hoping to raise the number of doses in its vials to as many as 15 from the current 10 doses, a potential 50 percent increase. The proposal reflects the fact that the company has been ramping up production of its vaccine to the point where the final manufacturing stage, when it is bottled, capped and labeled, has emerged as a roadblock to expanding its distribution.
The FDA will decide in a few weeks???! That is not quick, bold, and decisive action.
Thousands of people have already died over a few weeks delay–multiple times. It’s true that there are tradeoffs but the FDA has no special knowledge or ability to navigate those tradeoffs. Let Moderna make the decision in consultation with vaccinators on the front lines.
Thwarted markets in everything
Philadelphia health officials say they’re no longer providing vaccines to a 9-month-old start-up that has begun inoculating thousands of city residents, citing the group’s quiet switch to a for-profit entity.
“We have recently been made aware of a change in Philly Fighting COVID’s corporate status that took place without our knowledge, from nonprofit to for-profit,” said Health Department spokesperson Jim Garrow.
The move comes days after WHYY News and Billy Penn reported that Philly Fighting COVID had established a for-profit arm, and that when the group pivoted from providing community testing to performing vaccinations, it left several partner organizations in the lurch.
Here is the full story.
Markets in everything
Illicit sales of fake negative Covid-19 test results are becoming more widespread as criminals look to profit from travel restrictions imposed during the pandemic, according to Europol.
The EU’s law enforcement agency on Monday reported an increase in cases of fraudulent Covid-19 test certificates being sold to travelers. It comes as an increasing number of countries in the European Union and beyond oblige travelers to present a negative coronavirus test in order to be allowed entry, when travelling from a high-risk area.
In its latest Early Warning Notification, which Europol issues to alert EU member states of new or increasingly prevalent forms of criminal activity, the agency said the latest case of this crime had been detected in Luton Airport in the U.K., where a man was arrested trying to sell false coronavirus test results. Elsewhere in the U.K., fraudsters were caught selling bogus Covid-19 test documents for £100 ($137).
There had also been earlier reports of similar activity in other European countries.
A forgery ring at Charles de Gaulle Airport in Paris, for example, was “dismantled” after being found selling forged negative test results to passengers, Europol said. The amount charged for the fake test documents ranged between 150-300 euros ($181-$363).
Another fraudster was apprehended in Spain for selling false negative test certificates on the internet for 40 euros, and in the Netherlands, scammers were discovered selling fake negative test results for 50-60 euros through messaging apps.
Here is the full article, via Samarth.
The Experts are Very Worried
Here is an interview with Dr. Peter Hotez, dean of the National School of Tropical Medicine at Baylor College of Medicine and the lead developer of a COVID vaccine being produced in India. He thinks the AstraZeneca vaccine should be approved immediately, as I have long argued.
President Biden himself announced Tuesday that we’re going to have maybe enough additional doses of the mRNA vaccines to fully vaccinate 300 million Americans by the end of summer or fall.
I’m saying, “Well, no, that’s that’s not gonna work.” Telling us “by the fall” is like telling us “when the glaciers are gonna come back down from Quebec.” I mean, that’s not adequate.
We’re going to have to figure out a way to vaccinate the American people by late spring. That’s a tall order. To beat back the virus we need to give two doses to three-quarters of the population, to 246 million Americans. That’s half a billion immunizations. To get there, we’d need a rate of immunizations two or three times higher than what’s proposed.
….We need vaccinations now.
..things have been slowed down with the AstraZeneca-Oxford adenovirus vaccine. My understanding is that the FDA insisted that they conduct a full-scale Phase Three trial in the U.S., and we won’t have results for that until April. Meanwhile, the European Medicines Agency, the EMA, is going to make a ruling on the AstraZeneca Oxford vaccine on Friday based on studies done in Europe and also probably on data from Brazil and South Africa. [The EMA authorized, AT].
Those are large, reliable studies?
Yeah….Because of these new variants, there’s great urgency here in the U.S. So I’m saying that sometimes we have to do things that take us out of our comfort zone in order to save lives. That means, rather than focusing only on the new study that we’re doing in the U.S., we also look at the dossier presented to the EMA.
As a regulatory agency the EMA is up there with our U.S. FDA. They’re the two best regulatory agencies in the world. So if they sign off, I think we should say, “Look, let’s do it. Let’s use that vaccine.”
We’ve already bought 300 million doses of the AstraZeneca-Oxford vaccine. We’ve paid for it — over a billion dollars — so let’s use it.
…And there’s also the recombinant protein vaccine our lab has developed at Baylor College of Medicine and Texas Children’s Hospital. In India they’re scaling that up to a billion doses. Nobody from the White House has approached us to say, “Hey, Peter, what can we do to bring that vaccine in.”
There seem to be blinders: All they can see is getting the mRNA vaccines. I don’t quite know what’s driving that. We have to figure out a way to bring the other ones on board.
And soon! We’re in the eye of the hurricane.
Hat tip: Jim Ward.
Scott Sumner on Vaccine Nationalism
EconLib: Experts in the UK have looked at the AstraZenaca vaccine and found it to be safe and effective. And yet Americans are still not allowed to use the product. So if paternalism is not the actual motive, why do progressives insist that Americans must not be allowed to buy products not approved by the FDA? What is the actual motive?
The answer is nationalism. The experts who studied the AstraZenaca vaccine were not American experts, they were British experts. Can this form of prejudice be justified on scientific grounds? Obviously not. There has been no double blind, controlled study of comparative expert skill at evaluating vaccines. We have no way of knowing whether the UK decision is wiser than the FDA decision. Instead, the legal prohibition is being done on nationalistic grounds. We are told to blindly accept the incompetence of British experts, without any proof. (And even if you believed there was solid evidence that one country’s experts were better than another, it would not explain why each developed countries relies on their own experts. They can’t all be best!)
These debates always end up being like a game of whack-a-mole. Shoot down one argument and regulation proponents will simply put forth another. Their minds are made up. You say people shouldn’t be allowed to take a vaccine unless experts find it to be safe and effective? OK, the UK experts did just that. You say that only the opinion of US experts counts because our experts are clearly the best? Really, where is the scientific study that shows that our experts are the best? I thought you said we needed to “trust the scientists”? Now you are saying we must trust the nationalists?
…what’s wrong with the following three-part system of regulation as a compromise solution:
1. FDA approved drugs can be consumed by anyone in America.
2. Drugs approved by any of the top 20 advanced countries (but not the FDA) can be consumed by anyone willing to sign a consent form indicating that they understand the FDA has not approved this product. I’ll sign for AstraZeneca. (The US government puts together a list of 20 reputable countries.)
3. Drugs approved by none of the top 20 developed economies will still be banned.
This is what regulation would look like if paternalism actually were the motivating factor. But it’s not. It’s Trump-style nationalism that motivates progressives to insist that only FDA approved drugs can be sold in America. They may look down their noses at Trump, but they implicitly share his nationalism.
I agree, of course, and have long supported Pharmaceutical Reciprocity.